Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Properties of Colloids
PROPERTIES OF COLLOIDS
The properties of colloids are discussed under
three types
i.
Kinetic property
ii.
Optical property
iii.
Electrical property
(i) Kinetic property
When sol
is examined with an ultramicrosope, the suspended particles are seen as shining
specks of light. By following an individual particle, it is observed that the
particle is undergoing a constant rapid motion. It moves in a series short
straight line paths in the medium, changing direction abruptly.
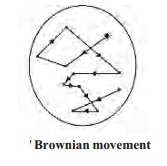
The
continuous rapid zig-zag chaotic random and ceaseless movement executed by a
colloidal particle in the dispersion medium is called brownian movement. This
is due to the unbalanced bombardment of the particles by the molecules of the
dispersion medium.
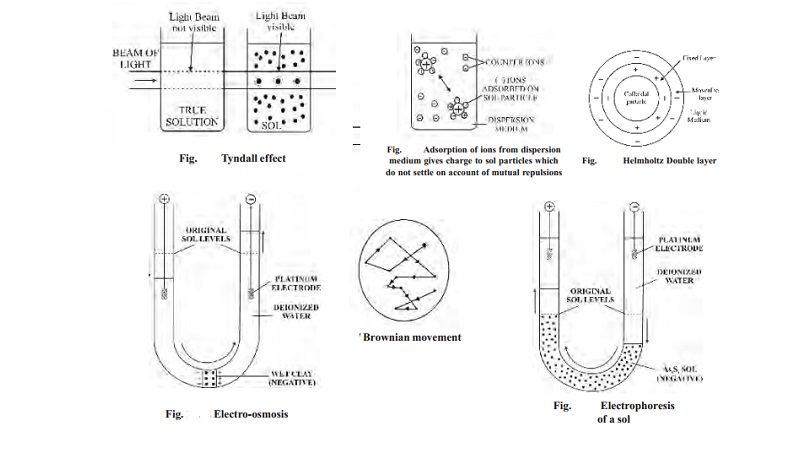
(ii) Optical property
When a
strong beam of light is passed through a sol and viewed at right angles, the
path of light shows up as a hazy beam. This is due to the fact that sol
particles absorb light energy and then emit it in all directions. This
scattering of light illuminates the path of the beam. The phenomenon of the
scattering of light by the sol particles is called Tyndall effect.
(iii). Electrical
Properties
(i) Charge on
Colloidal particles
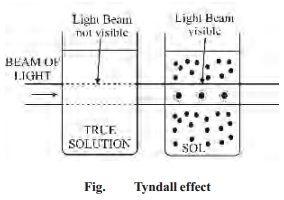
The
important property of colloidal dispersions is that all the suspended particles
possess either a positive or negative charge. The mutual forces of repulsion
between similarly charged particles prevent them from aggregating and settling
under the action of gravity. This gives stability to the sol.
The surface of colloidal particle acquires a
positive charges by selective adsorption of a layer of positive ions around it.
This layer attracts counterions from the medium which form a second layer of
negative charges. The combination of the two layers of charges around the sol
particle is called Helmholtz double
layer.
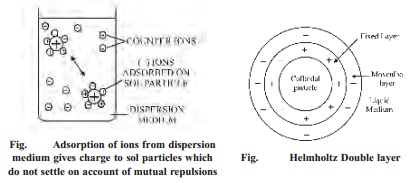
(ii) Electrophoresis
If
electric potential is applied across two platinum electrodes immersed in a
hydrophilic sol, the dispersed particles move toward one or the other
electrode. The movement of sol particles under an applied electric potential is
called electrophoresis or cataphoresis. If the sol particles here negatively
charged, they migrate toward the positive electrode. On the other hand, if they
have positively charged they move toward the negative electrode. From the
direction of movement of the sol particles, we can determine the charge of the
sol particles.
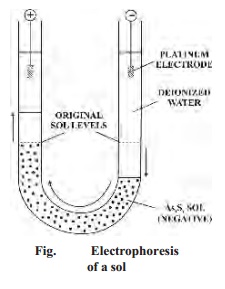
The
phenomenon of electrophoresis can be demonstrated by placing a layer of As2S3
sol under two limbs of a U-tube. When a potential difference of about 100 volts
is applied across the two platinum electrodes dipping in deionised water, it is
observed that the level of the sol drops on the negative electrode side and
rises on the positive electrode side (Fig.) This shows that As2S3
sol has migrated to the positive electrode, indicating that the particles are
negatively charged.
(iii) Electro osmosis
In a sol,
the dispersion medium carries an equal but opposite charge to that of the
dispersed particles. Thus, the medium will move in opposite direction to the
dispersed phase under the influence of applied electric potential. The movement
of the dispersion medium under the influence of applied potential is known as
electro-osmosis.

The
phenomenon of electro-osmosis can be demonstrated by using a U-tube in which a
plug of wet clay (a negative colloid) is fixed. The two limbs of the tube are
filled with water to the same level. The platinum electrodes are immersed in
water and potential applied across them. It will be observed that water level rises
on the cathode side and falls on anode side. This movement of the medium
towards the negative electrode, shows that the charge on the medium is
positive. Similarly, for a positively charged colloid electro-osmosis will take
place in the reverse direction.
Related Topics