Chapter: Biochemistry: The Behavior of Proteins: Enzymes
Practical Information from Kinetic Data
Practical Information from Kinetic Data
The mathematics of enzyme kinetics can certainly look
challenging. In fact, an understanding of kinetic parameters can often provide
key information about the role of an enzyme within a living organism. Many of
the ways of doing kinetic plots of this sort were developed by physical organic
chemists, who then went on to propose mechanisms for reactions of all sorts
based on kinetic data. Four aspects are useful: comparison of KM, comparison of kcat or turnover
number, comparison of kcat/ KMratios, and speci√ěc locations of enzymes
within an organism.
Comparison of KM
Let us start by comparing the values of KM for two enzymes
that catalyze an early step in the breakdown of sugars: hexokinase and
glucokinase. Both enzymes catalyze the formation of a phosphate ester linkage
to a hydroxyl group of a sugar. Hexokinase can use any one of several
six-carbon sugars, including glucose and fructose, the two components of
sucrose (common table sugar), as substrates. Glucokinase is an isozyme of
hexokinase that is primarily involved in glucose metabolism. The KM for hexokinase
is 0.15 mM for glucose and 1.5 mM for fructose.
The KM for glucokinase, a liver-specific enzyme, is 20 mM. (We
shall use the expression KM here, even though some hexokinases
studied do not follow Michaelis-Menten kinetics, and the term [S]0.5 might be more
appropriate. Not all enzymes have a KM, but they do all have a substrate
concentration that gives rise to Vmax/2.
Comparison of these numbers tells us a lot about sugar
metabolism. Because the resting level for blood glucose is about 5 mM, hexokinase
would be expected to be fully active for all body cells. The liver would not be
competing with the other cells for glucose. However, after a carbohydrate-rich
meal, blood glu-cose levels often exceed 10 mM, and, at that
concentration, the liver glucokinase would have reasonable activity. Furthermore,
because the enzyme is found only in the liver, the excess glucose will be
preferentially taken into the liver, where it can be stored as glycogen until
it is needed. Also, the comparison of the two sugars for hexokinase indicates
clearly that glucose is preferred over fructose as a nutrient.
Similarly, if one compares the form of the enzyme lactate
dehy-drogenase found in heart muscle to the type found in skeletal muscle, one
can see small differences in amino acid composition. These differences in turn
affect the reaction catalyzed by this enzyme, the conversion of pyruvate to
lactate. The heart type has a high KM, or a low affinity for
pyruvate, and the muscle type has a low KM, or a high affinity
for pyruvate. This means that the pyruvate will be preferentially converted to
lactate in the muscle but will be preferentially used for aerobic metabolism in
the heart, rather than being converted to lactate. These conclusions are
consistent with the known biology and metabolism of these two tissues.
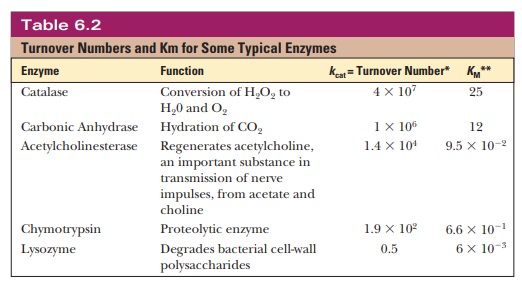
Comparison of Turnover Number
As can be seen from Table 6.2, the √ěrst two enzymes are very
reactive; catalase has one of the highest turnover numbers of all known
enzymes. These high numbers allude to their importance in detoxifying hydrogen
peroxide and preventing formation of CO2 bubbles in the blood; these are their
respective reactions.
Related Topics