Chapter: Biochemistry: The Behavior of Proteins: Enzymes, Mechanisms, and Control
Zymogens
Zymogens
Allosteric
interactions control the behavior of proteins through reversible changes in
quaternary structure, but this mechanism, effective though it may be, is not
the only one available. A zymogen,
an inactive precursor of an enzyme, can be irreversibly transformed into an
active enzyme by cleavage of covalent bonds.
The
proteolytic enzymes trypsin and chymotrypsin provide a classic example of
zymogens and their activation. Their inactive precursor molecules, trypsinogen
and chymotrypsinogen, respectively, are formed in the pancreas, where they
would do damage if they were in an active form. In the small intestine, where
their digestive properties are needed, they are activated by cleavage of specific
peptide bonds. The conversion of chymotrypsinogen to chymotrypsin is catalyzed
by trypsin, which in turn arises from trypsinogen as a result of a cleavage
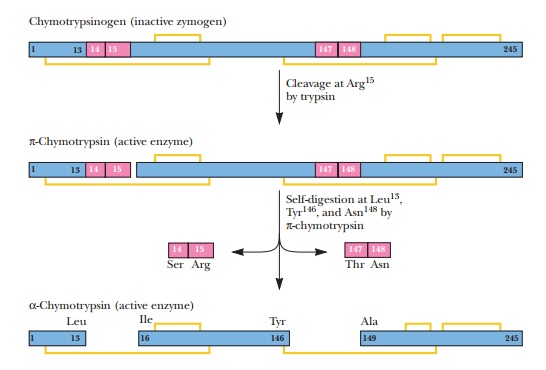
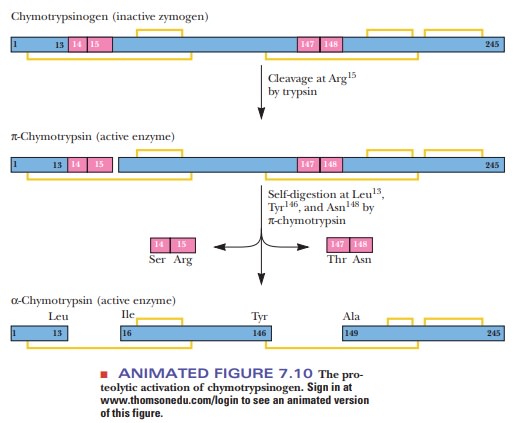
reaction catalyzed by the enzyme enteropeptidase. Chymotrypsinogen consists of
a single polypeptide chain 245 residues long, with five disulfide (-S-S-)
bonds. When chymotrypsinogen is secreted into the small intestine, trypsin
present in the digestive system cleaves the peptide bond between arginine 15
and isoleucine 16, counting from the N-terminal end of the chymotrypsinogen
sequence (Figure 7.10). The cleavage produces active p-chymotrypsin. The
15-residue fragment remains bound to the rest of the protein by a disulfide
bond. Although p-chymotrypsin is fully active, it is not the end product of this
series of reactions. It acts on itself to remove two dipeptide fragments,
producing α-chymotrypsin, which is also fully active. The two dipeptide
fragments cleaved off are Ser 14-Arg 15 and Thr 147-Asn 148; the final form of
the enzyme, α-chymotrypsin, has three polypeptide chains held together by two
of the five original, and still intact, disulfide bonds. (The other three
disulfide bonds remain intact as well; they link portions of single polypeptide
chains.) When the term chymotrypsin
is used without specifying the a or the p form, the final a form is meant.
The changes in primary structure that accompany the conversion of chymo-trypsinogen to α-chymotrypsin bring about changes in the tertiary structure. The enzyme is active because of its tertiary structure, just as the zymogen is inactive because of its tertiary structure. The three-dimensional structure of chymotrypsin has been determined by X-ray crystallography.
The protonated amino group of the
isoleucine residue exposed by the first cleavage reaction is involved in an
ionic bond with the carboxylate side chain of aspartate residue
This ionic bond is necessary
for the active conformation of the enzyme because it is near the active site.
Chymotrypsinogen lacks this bond; therefore, it does not have the active
conformation and cannot bind substrate.
Blood
clotting also requires a series of proteolytic activations involving sev-eral
proteins, particularly the conversions of prothrombin to thrombin and of
fibrinogen to fibrin. Blood clotting is a complex process; for this discussion,
it is sufficient to know that activation of zymogens plays a crucial role. In
the final, best-characterized step of clot formation, the soluble protein
fibrinogen is con-verted to the insoluble protein fibrin as a result of the
cleavage of four peptide bonds. The cleavage occurs as the result of action of
the proteolytic enzyme thrombin, which, in turn, is produced from a zymogen
called prothrombin. The conversion of prothrombin to thrombin requires Ca2+ as well
as a number of proteins called clotting
factors.
Summary
Zymogens are inactive precursors of an enzyme.
A zymogen is converted to the active form by the irreversible
cleavage of specific peptide bonds in the protein.
Many
digestive enzymes, such as trypsin and chymotrypsin, are initially produced as
zymogens. They become active only after arriving at their final destination.
Related Topics