Chapter: Biochemistry: The Behavior of Proteins: Enzymes, Mechanisms, and Control
Control of Enzyme Activity by Phosphorylation
Control of Enzyme Activity by
Phosphorylation
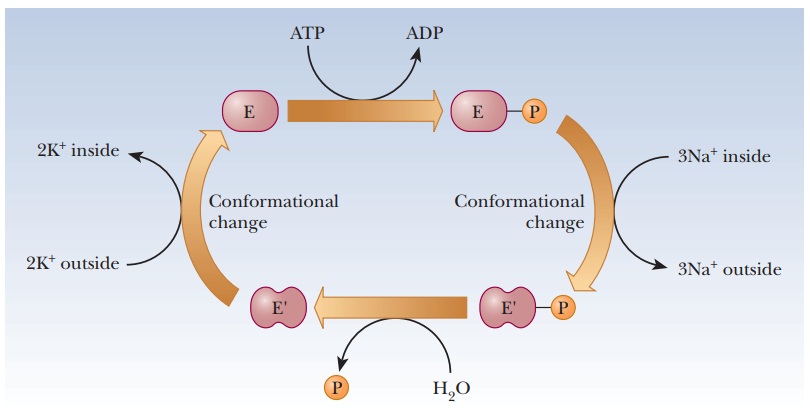
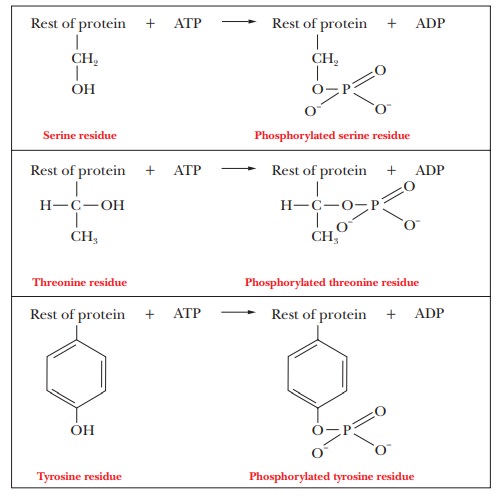
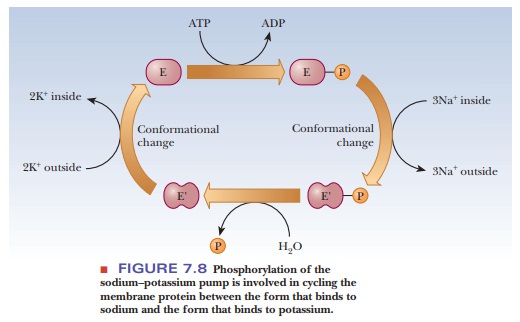
One of the most common control mechanisms for enzymes is by phosphorylation. The side-chain hydroxyl groups of serine, threonine, and tyrosine can all form phosphate esters. Transport across membranes provides an important example, such as the sodium–potassium ion pump, which moves potassium into the cell and sodium out. The source of the phosphate group for the protein component of the sodium–potassium ion pump and for many enzyme phosphorylations is the ubiquitous ATP. When ATP is hydrolyzed to adenosine diphosphate (ADP), enough energy is released to allow a number of otherwise energetically unfavorable reactions to take place. In the case of the Na+/K+ pump, ATP donates a phosphate to aspartate 369 as part of the mechanism, causing a conformation change in the enzyme (Figure 7.8). Proteins that catalyze these phosphorylation reactions are called protein kinases.Kinase refers to an enzyme that catalyzes transfer of a phosphate group, almost always from ATP, to some substrate. These enzymes play an important role in metabolism.
Many
examples appear in processes involved in generating energy, as is the case in
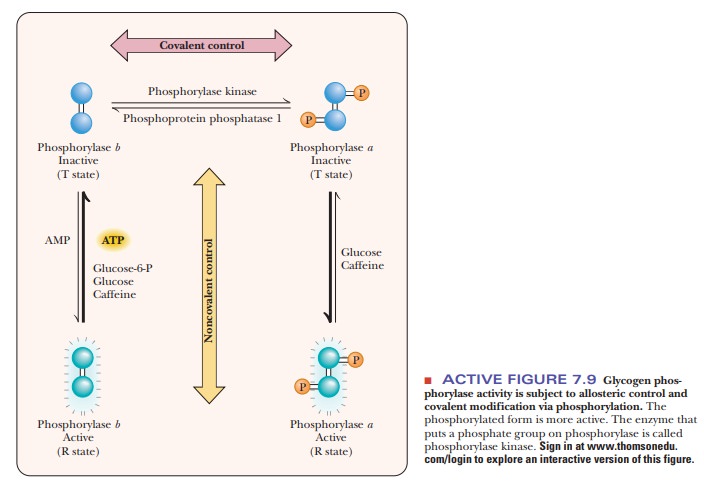
carbohydrate metabolism. Glycogen phosphorylase, which catalyzes the initial
step in the breakdown of stored glycogen, exists in two forms-the
phosphorylated glycogen phosphorylase a
and the dephosphory-lated glycogen phosphorylase b (Figure 7.9). The a
form is more active than the b form,
and the two forms of the enzyme respond to different allosteric effectors,
depending on tissue type. Glycogen phosphorylase is thus subject to two kinds
of control-allosteric regulation and covalent modification. The net result is
that the a form is more abundant and
active when phosphorylase is needed to break down glycogen to provide energy.
Does phosphorylation always increase enzyme activity?
Although
it would be convenient to have a model in which phosphorylation always
increases the activity of an enzyme, biochemistry is not so kind to us. In
reality, we cannot predict whether phosphorylation will increase or decrease
the activity of an enzyme. In some systems, the effects on two opposing enzymes
are coordinated. For example, a key enzyme in a catabolic pathway may be
activated by phosphorylation while its counterpart in an anabolic opposing
pathway is inhibited by phosphorylation.
Summary
Many enzymes are controlled by phosphorylation.
Enzymes called kinases use high-energy molecules, such as ATP, to
trans-fer a phosphate to a specific residue in an enzyme.
These amino acid residues are usually serine, threonine, or
tyrosine residues.
In some
cases, phosphorylation increases the activity of an enzyme, while in other
cases it decreases it.
Related Topics