Chapter: Biochemistry: The Behavior of Proteins: Enzymes, Mechanisms, and Control
The Concerted and Sequential Models for Allosteric Enzymes
The Concerted and Sequential
Models for Allosteric Enzymes
The two
principal models for the behavior of allosteric enzymes are the concerted model
and the sequential model. They were proposed in 1965 and 1966, respectively,
and both are currently used as a basis for interpreting experimental results.
The concerted model has the advantage of comparative simplicity, and it
describes the behavior of some enzyme systems very well.
The
sequential model sacrifices a certain amount of simplicity for a more realistic
picture of the structure and behavior of proteins; it also deals very well with
the behavior of some enzyme systems.
What is the concerted model for allosteric behavior?
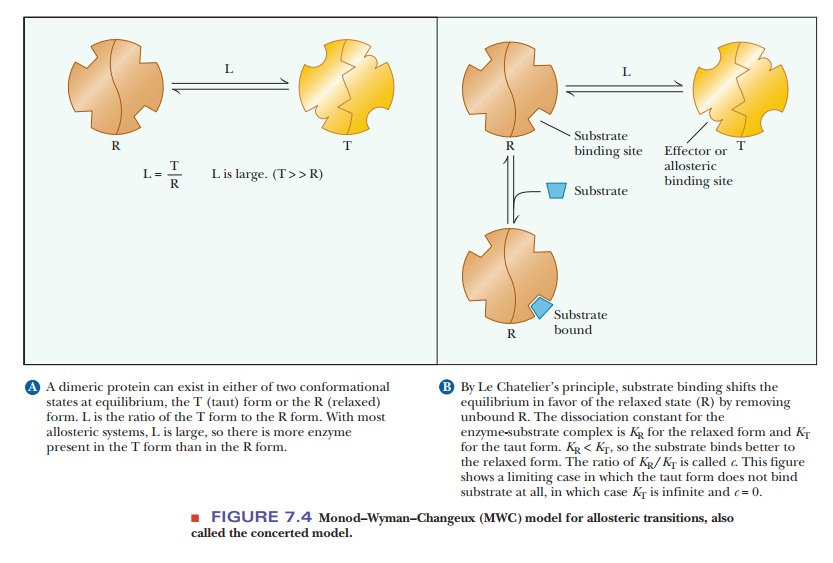
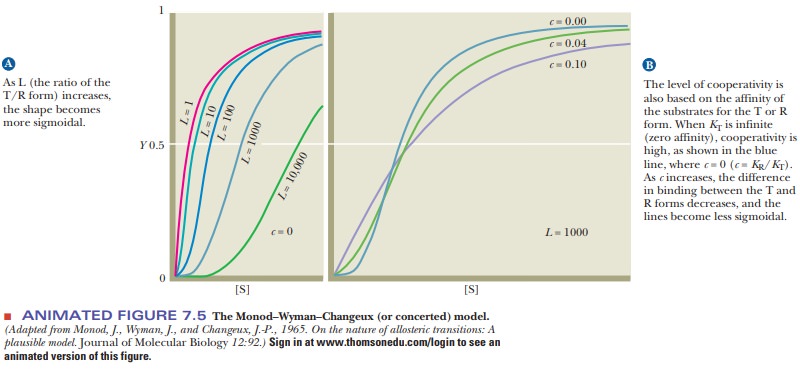
In 1965, Jacques Monod, Jeffries Wyman, and Jean-Pierre Changeux proposed the concerted model for the behavior of allosteric proteins in a paper that has become a classic in the biochemical literature. In this picture, the protein has two conformations, the active R (relaxed) conformation, which binds substrate tightly, and the inactive T (tight, also called taut) conformation, which binds substrate less tightly. The distinguishing feature of this model is that the conformations of all subunits change simultaneously. Figure 7.4a shows a hypothetical protein with two subunits. Both subunits change conformation from the inactive T conformation to the active R conformation at the same time; that is, a concerted change of conformation occurs. The equilibrium ratio of the T/R forms is called L and is assumed to be high-that is, more enzyme is present in the unbound T form than in the unbound R form. The binding of substrate to either form can be described by the dissociation constant of the enzyme and substrate, K, with the affinity for substrate higher in the R form than in the T form. Thus, KR<<KT. The ratio of KR/KT is called c. Figure 7.4b shows a limiting case in which KTis infinitely greater than KR(c = 0). In other words, substrate will not bindto the T form at all. The allosteric effect is explained by this model based on perturbing the equilibrium between the T and R forms. Although initially the amount of enzyme in the R form is small, when substrate binds to the R form, it removes free R form. This causes the production of more R form to reestablish the equilibrium, which makes binding more substrate possible. This shifting of the equilibrium is responsible for the observed allosteric effects. The Monod–Wyman–Changeux model has been shown mathematically to explain the sigmoidal effects seen with allosteric enzymes. The shape of the curve will be based on the L and c values. As L increases (free T form more highly favored), the shape becomes more sigmoidal (Figure 7.5). As the value for c decreases (higher affinity between substrate and R form), the shape also becomes more sigmoidal.
In the concerted model, the effects of
inhibitors and activators can also be considered in terms of shifting the
equilibrium between the T and R forms of the enzyme. The binding of inhibitors
to allosteric enzymes is cooperative; allo-steric inhibitors bind to and
stabilize the T form of the enzyme. The binding of activators to allosteric
enzymes is also cooperative; allosteric activators bind to and stabilize the R
form of the enzyme. When an activator, A, is present, the cooperative binding
of A shifts the equilibrium between the T and R forms, with the R form favored
(Figure 7.6). As a result, there is less need for substrate, S, to shift the
equilibrium in favor of the R form, and less cooperativity in the binding of S
is seen.
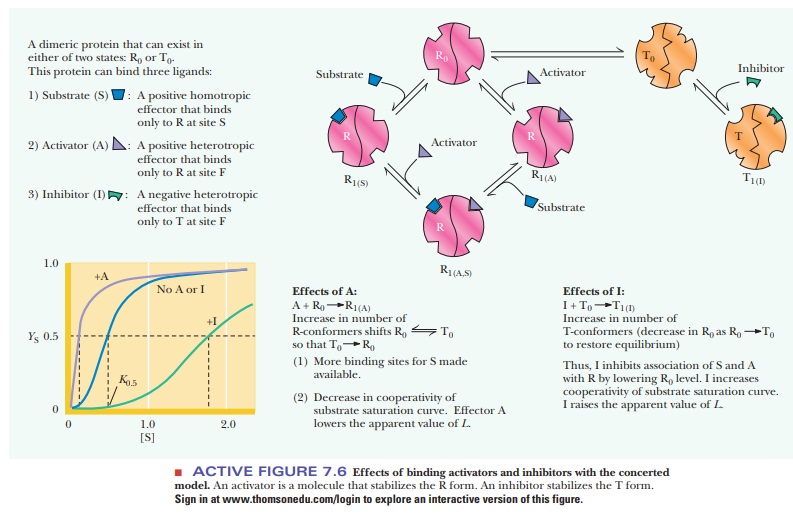
When an inhibitor, I, is present, the
cooperative binding of I also shifts the equilibrium between the T and R forms,
but this time the T form is favored (Figure 7.6). More substrate is needed to
shift the T-to-R equilibrium in favor of the R form. A greater degree of
cooperativity is seen in the binding of S.
What is the sequential model for allosteric behavior?
The name
Daniel Koshland is associated with the direct sequential model of allosteric behavior. The distinguishing feature
of this model is that the binding of substrate induces the conformational
change from the T form to the R form-the type of behavior postulated by the
induced-fit theory of substrate binding. A conformational change from T to R in
one subunit makes the same conformational change easier in another subunit, and
this is the form in which cooperative binding is expressed in this model
(Figure 7.7a).
In the sequential model, the binding of activators and inhibitors also takes place by the induced-fit mechanism. The conformational change that begins with binding of inhibitor or activator to one subunit affects the conformations of other subunits. The net result is to favor the R state when activator is present and to favor the T form when inhibitor, I, is present (Figure 7.7b).
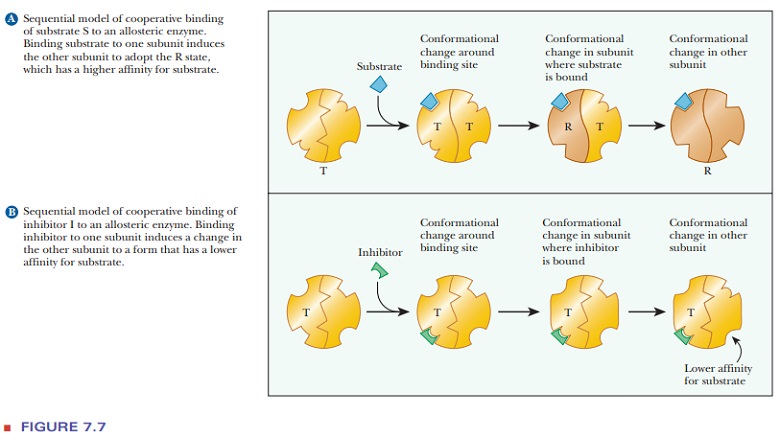
Binding I to one
subunit causes a conformational change such that the T form is even less likely
to bind substrate than before. This conformational change is passed along to
other subunits, making them also more likely to bind inhibitor and less likely
to bind substrate. This is an example of cooperative behavior that leads to more
inhibition of the enzyme. Likewise, binding an activator causes a
conformational change that favors substrate binding, and this effect is passed
from one subunit to another.
The
sequential model for binding effectors of all types, including substrates, to allosteric
enzymes has a unique feature not seen in the concerted model. The
conformational changes thus induced can make the enzyme less likely to bind
more molecules of the same type. This phenomenon, called negativecooperativity, has been observed in a few enzymes. One is
tyrosyl tRNA synthe-tase, which plays a role in protein synthesis. In the
reaction catalyzed by this enzyme, the amino acid tyrosine forms a covalent
bond to a molecule of trans-fer RNA (tRNA). In subsequent steps, the tyrosine
is passed along to its place in the sequence of the growing protein. The
tyrosyl tRNA synthetase consists of two subunits. Binding of the first molecule
of substrate to one of the subunits inhibits binding of a second molecule to
the other subunit.
The
sequential model has successfully accounted for the negative coopera-tivity
observed in the behavior of tyrosyl tRNA synthetase. The concerted model makes
no provision for negative cooperativity.
Summary
The two principal models for allosteric enzyme behavior are called
the concerted model and the sequential model.
In the concerted model, the enzyme is thought
of as being in a taut form, T, or a relaxed form, R. All subunits are found in
one or the other, and an equilibrium exists between the T and R forms.
Substrate binds more easily to the R form than to the T form,
inhibitors stabilize the T form, and activators stabilize the R form.
In the sequential model, subunits of the enzyme can change
sequentially from the T form to the R form and back again.
Binding of one molecule of substrate to one
subunit stimulates the transi-tion of the subunit to the R form, which then
stimulates another subunit to change to the R form.
Binding
of inhibitor to one subunit induces a change in the other sub-units to a form
with lower affinity for the substrate. Binding of an activator to one subunit
induces a shift in the other subunits to a form that has a high affinity for
substrate.
Related Topics