Chapter: Biochemistry: The Behavior of Proteins: Enzymes, Mechanisms, and Control
The Behavior of Allosteric Enzymes
The Behavior of Allosteric
Enzymes
The
behavior of many well-known enzymes can be described quite adequately by the
Michaelis–Menten model, but allosteric enzymes behave very differently. We saw
similarities between the reaction kinetics of an enzyme such as chymotrypsin,
which does not display allosteric behavior, and the binding of oxygen by
myoglobin, which is also an example of nonallosteric behavior. The analogy
extends to show the similarity in the kinetic behavior of an allosteric enzyme
such as aspartate transcarbamoylase (ATCase) and the binding of oxygen by
hemoglobin. Both ATCase and hemoglobin are allosteric proteins; the behaviors
of both exhibit cooperative effects caused by subtle changes in quaternary
structure. (Recall that quaternary
structure is the arrangement in space that results from the interaction of
subunits through noncovalent forces, and that positive cooperativity refers to the fact that the binding of low
levels of substrate facilitates the action of the protein at higher levels of
substrate, whether the action is catalytic or some other kind of binding.) In
addition to displaying cooperative kinetics, allosteric enzymes have a
different response to the presence of inhibitors from that of nonallosteric
enzymes.
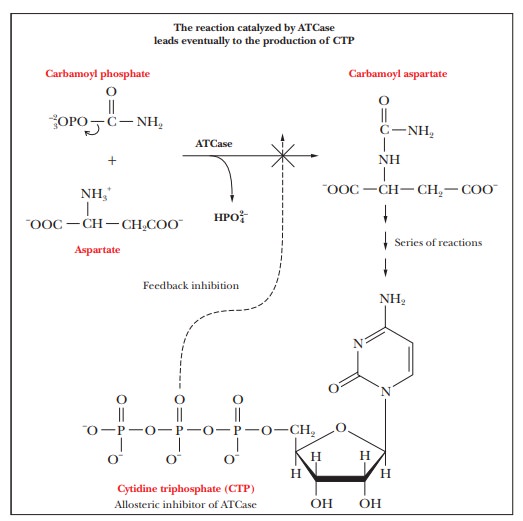
How are allosteric enzymes controlled?
ATCase
catalyzes the first step in a series of reactions in which the end product is
cytidine triphosphate (CTP), a nucleoside triphosphate needed to make RNA and
DNA. The pathways that produce nucleotides are energetically costly and involve
many steps. The reaction catalyzed by aspartate transcarbamoylase is a good
example of how such a pathway is controlled to avoid overproduction of such
compounds. For DNA and RNA synthesis, the levels of several nucleotide
triphosphates are controlled. CTP is an inhibitor of ATCase, the enzyme that
catalyzes the first reaction in the pathway. This behaviour is an example of feedback inhibition (also called
end-product inhibition), in which the
end product of the sequence of reactions inhibits the first reaction in the
series (Figure 7.1). Feedback inhibition is an efficient control mechanism because the entire series of reactions can be
shut down when an excess of the final product exists, thus preventing the
accumulation of intermediates in the pathway. Feedback inhibition is a general
feature of metabolism and is not confined to allosteric enzymes. However, the
observed kinetics of the ATCase reaction, including the mode of inhibition, are
typical of allosteric enzymes.

When
ATCase catalyzes the condensation of aspartate and carbamoyl phosphate to form
carbamoyl aspartate, the graphical representation of the rate as a function of
increasing substrate concentration (aspartate) is a sigmoidal curve rather than
the hyperbola obtained with nonallosteric enzymes (Figure 7.2a). The sigmoidal
curve indicates the cooperative behavior of allosteric enzymes. In this
two-substrate reaction, aspartate is the substrate for which the concentration
is varied, while the concentration of carbamoyl phosphate is kept constant at
high levels.
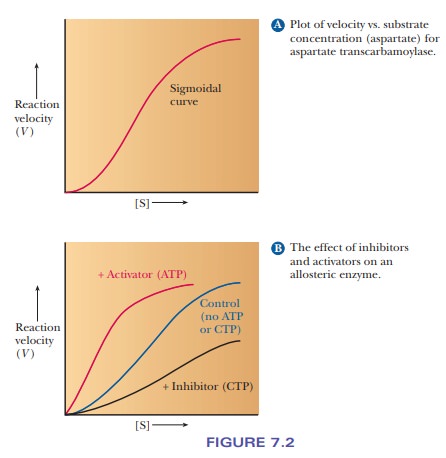
Figure
7.2b compares the rate of the uninhibited reaction of ATCase with the reaction
rate in the presence of CTP. In the latter case, a sigmoidal curve still
describes the rate behavior of the enzyme, but the curve is shifted to higher substrate
levels; a higher concentration of aspartate is needed for the enzyme to achieve
the same rate of reaction. At high substrate concentrations, the same maximal
rate, Vmax, is
observed in the presence and absence of inhibitor.
Because in the Michaelis–Menten scheme Vmax changes
when a reaction takes place in the presence of a noncompetitive inhib-itor,
noncompetitive inhibition cannot be the case here. The same Michaelis– Menten
model associates this sort of behavior with competitive inhibition, but that
part of the model still does not provide a reasonable picture. Competitive
inhibitors bind to the same site as the substrate because they are very similar
in structure. The CTP molecule is very different
in structure from the substrate, aspartate, and it is bound to a different site
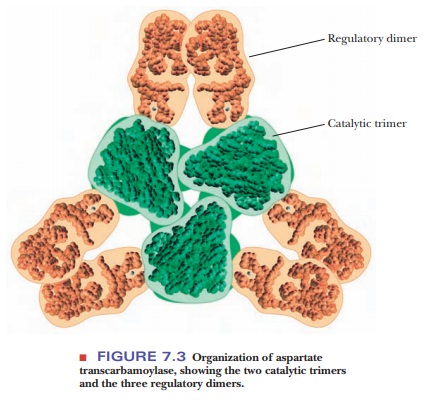
on the ATCase molecule. ATCase is made up of two different types of subunits.
One of them is the catalytic subunit, which consists of six protein subunits
organized into two trimers. The other is the regulatory subunit, which also
consists of six protein subunits organized into three dimers (Figure 7.3). The
catalytic subunits can be separated from the regulatory subunits by treatment
with p-hydroxymercuribenzoate, which
reacts with the cysteines in the protein. When so treated, ATCase still
catalyzes the reaction, but it loses its allosteric control by CTP, and the
curve becomes hyperbolic.
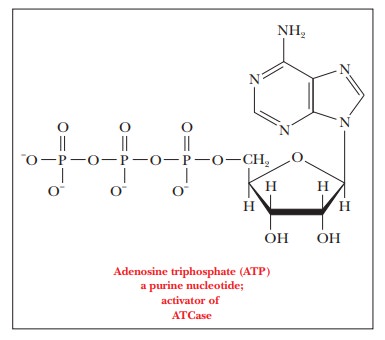
The situation becomes “curiouser and curiouser” when the ATCase reaction takes place not in the presence of CTP, a pyrimidine nucleoside triphosphate, but in the presence of adenosine triphosphate (ATP), a purine nucleoside tri-phosphate. The structural similarities between CTP and ATP are apparent, but ATP is not a product of the pathway that includes the reaction of ATCase and that produces CTP. Both ATP and CTP are needed for the synthesis of RNA and DNA. The relative proportions of ATP and CTP are specified by the needs of the organism. If there is not enough CTP relative to the amount of ATP, the enzyme requires a signal to produce more.
In the presence of ATP, the rate of the enzymatic reaction is increased at lower levels of aspartate, and the shape of the rate curve becomes less sigmoidal and more hyperbolic (Figure 7.2b). In other words, there is less cooperativity in the reaction. The binding site for ATP on the enzyme molecule is the same as that for CTP (which is not surpris-ing in view of their structural similarity), but ATP is an activator rather than an inhibitor like CTP. When CTP is in short supply in an organism, the ATCase reaction is not inhibited, and the binding of ATP increases the activity of the enzyme still more.
Even
though it is tempting to consider inhibition of allosteric enzymes in the same
fashion as nonallosteric enzymes, much of the terminology is not appro-priate. Competitive inhibition and noncompetitive inhibition are terms
reserved for the enzymes that behave in line with Michaelis–Menten kinetics.
With allosteric enzymes, the situation is more complex. In general, two types
of enzyme sys-tems exist, called K
systems and V systems. A K
system is an enzyme for which the substrate concentration that yields one-half Vmax is
altered by the presence of inhibitors or activators. ATCase is an example of a
K system. Because we are not dealing with a Michaelis–Menten type of enzyme,
the term KM is not
appli-cable. For an allosteric enzyme, the substrate level at one-half Vmax is
called the K0.5. In a V
system, the effect of inhibitors and activators changes theVmax, butnot the K0.5.
The key
to allosteric behavior, including cooperativity and modifications of
cooperativity, is the existence of multiple forms for the quaternary structures
of allosteric proteins. The word allosteric
is derived from allo, “other,” and steric, “shape,” referring to the fact
that the possible conformations affect the behav-ior of the protein. The
binding of substrates, inhibitors, and activators changes the quaternary
structure of allosteric proteins, and the changes in structure are reflected in
the behavior of those proteins. A substance that modifies the quaternary
structure, and thus the behavior, of an allosteric protein by binding to it is
called an allosteric effector. The
term effector can apply to
substrates, inhibitors, or activators. Several models for the behavior of
allosteric enzymes have been proposed, and it is worthwhile to compare them.
Let us
first define two terms. Homotropic
effects are allosteric interactions that occur when several identical molecules
are bound to a protein. The bind-ing of substrate molecules to different sites
on an enzyme, such as the binding of aspartate to ATCase, is an example of a
homotropic effect. Heterotropic
effects are allosteric interactions that occur when different substances (such
as inhibitor and substrate) are bound to the protein. In the ATCase reaction,
inhibition by CTP and activation by ATP are both heterotropic effects.
Summary
Allosteric enzymes exhibit different behaviors compared to nonallosteric
enzymes, and the Michaelis–Menten equations are not applicable.
A plot of velocity versus [S] for an allosteric enzyme has a
sigmoidal shape.
One type of control often seen with allosteric enzymes is called
feedback inhibition.
Inhibitors
and activators can control the activity of an allosteric enzyme.
Related Topics