Chapter: Biochemistry: The Behavior of Proteins: Enzymes, Mechanisms, and Control
Chemical Reactions Involved in Enzyme Mechanisms
Chemical Reactions Involved in
Enzyme Mechanisms
The
overall mechanism for a reaction may be fairly complex, as we have seen in the
case of chymotrypsin, but the individual parts of a complex mechanism can
themselves be fairly simple. Concepts such as nucleophilic attack and acid
catalysis commonly enter into discussions of enzymatic reactions. We can draw
quite a few general conclusions from these two general descriptions.
What are the most common types of reactions?
Nucleophilic substitution reactions play a
large role in the study of organicchemistry, and they are excellent
illustrations of the importance of kinetic measurements in determining the
mechanism of a reaction. A nucleophile is an electron-rich atom that attacks an
electron-deficient atom. A general equation for this type of reaction is
R:X + :Z -> R:Z + X
where :Z
is the nucleophile and X is called a leaving
group. In biochemistry, the carbon of a carbonyl group (C=O) is often the
atom attacked by the nucleophile. Common nucleophiles are the oxygens of
serine, threonine, and tyrosine. If the rate of the reaction shown here is
found to depend solely on the concentration of the R:X, then the nucleophilic
reaction is called an SN1 (substitution nucleophilic unimolecular). Such a mechanism would
mean that the slow part of the reaction is the breaking of the bond between R
and X, and that the addition of the nucleophile Z happens very quickly compared
to that. An SN1 reaction follows first-order kinetics. If the nucleophile attacks
the R:X while the X is still attached, then both the concentration of R:X and
the concentration of :Z will be important. This reaction will follow
second-order kinetics and is called an SN2 reaction (substitution nucleophilic bimolecular). The difference
between SN1 and SN2 is very important to
biochemists because it explains much about the stereospecificity of the
products formed. An SN1 reaction often leads to
loss of stereospecificity. Because the leaving group is gone before the
attacking group enters, the attacking group can often end up in one of two
orientations, although the specificity of the active site can also limit this.
With an SN2 reaction, the fact that the leaving group is still attached
forces the nucleophile to attack from a particular side of the bond, leading to
only one possible stereospecificity in the product. The chymotrypsin
nucleophilic attacks were examples of SN2 reactions,
although no stereochemistry is noted because the carbonyl that was attacked
became a carbonyl group again at the end of the reaction and was, therefore,
not chiral.
To
discuss acid–base catalysis, it is helpful to recall the definitions of acids
and bases. In the Brønsted–Lowry definition, an acid is a proton donor and a
base is a proton acceptor. The concept of general
acid–base catalysis depends on donation and acceptance of protons by groups
such as the imidazole, hydroxyl, carboxyl, sulfhydryl, amino, and phenolic side
chains of amino acids; all these functional groups can act as acids or bases.
The donation and accep-tance of protons gives rise to the bond breaking and
re-formation that consti-tute the enzymatic reaction.
If the
enzyme mechanism involves an amino acid donating a hydrogen ion, as in the
reaction
R-H+ + R-O– - > R
+ R-OH
then
that part of the mechanism would be called general acid catalysis. If an amino
acid takes a hydrogen ion from one of the substrates, such as in the reaction
R + R-OH - > R-H+ + R-O–
then
that part is called general base catalysis. Histidine is an amino acid that
often takes part in both reactions, because it has a reactive hydrogen on the
imidazole side chain that dissociates near physiological pH. In the chymotrypsin
mechanism, we saw both acid and base catalysis by histidine
A second
form of acid–base catalysis reflects another, more general defini-tion of acids
and bases. In the Lewis formulation, an acid is an electron-pair acceptor, and
a base is an electron-pair donor. Metal ions, including such biologically
important ones as Mn2+, Mg2+, and Zn2+, are
Lewis acids. Thus, they can play a role in metal–ion
catalysis (also called Lewis acid–base cataly-sis). The involvement of Zn2+ in the
enzymatic activity of carboxypeptidase A is an example of this type of
behavior. This enzyme catalyzes the hydrolysis of C terminal peptide bonds of
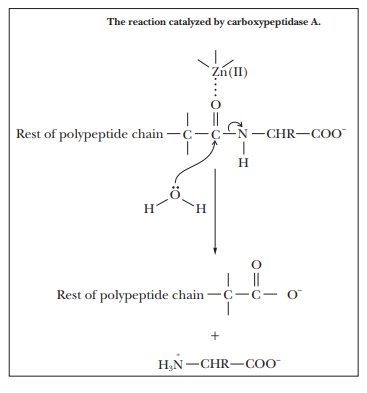
proteins. The Zn(II), which is required for the activity of the enzyme, is
complexed to the imidazole side chains of histidines 69 and 196 and to the
carboxylate side chain of glutamate 72. The zinc ion is also complexed to the
substrate.
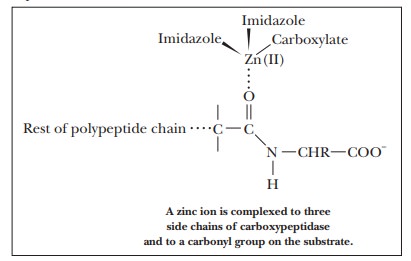
The type
of binding involved in the complex is similar to the binding that links iron to
the large ring involved in the heme group. Binding the substrate to the zinc
ion polarizes the carbonyl group, making it susceptible to attack by water and
allowing the hydrolysis to proceed more rapidly than it does in the uncatalyzed
reaction.
A
definite connection exists between the concepts of acids and bases and the idea
of nucleophiles and their complementary substances, electrophiles. A Lewis acid
is an electrophile, and a Lewis base is a nucleophile. Catalysis by enzymes,
including their remarkable specificity, is based on these well-known chemical
principles operating in a complex environment.
The nature of the active site plays a particularly important role in the speci-ficity of enzymes. An enzyme that displays absolute specificity, catalyzing the reac-tion of one, and only one, substrate to a particular product, is likely to have a fairly rigid active site that is best described by the lock-and-key model of sub-strate binding.
The many enzymes that display relative specificity, catalyzing the reactions of structurally related substrates to related products, apparently have more
flexibility in their active sites and are better characterized by the
induced-fit model of enzyme–substrate binding; chymotrypsin is a good example.
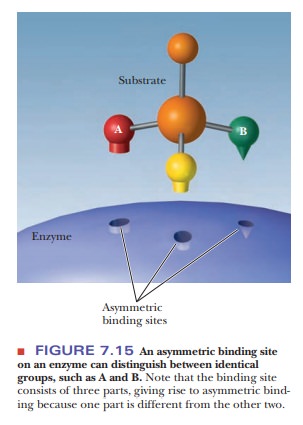
Finally, there are stereospecific
enzymes with specificity in which optical activity plays a role. The binding
site itself must be asymmetric in this situation (Figure 7.15). If the enzyme
is to bind specifically to an optically active substrate, the binding site must
have the shape of the substrate and not its mirror image. There are even
enzymes that introduce a center of optical activity into the product. The
substrate itself is not optically active in this case. There is only one
product, which is one of two possible isomers, not a mixture of optical
isomers.
Summary
Enzymes are known to catalyze familiar organic chemical reactions.
One of the most common is a nucleophilic substitution reaction, of
which there are two principal types-SN1 and SN2.
Other
common reactions are general acid–base catalysis and metal–ion catalysis.
Related Topics