Chapter: Biochemistry: The Behavior of Proteins: Enzymes
Examples of Enzyme-Catalyzed Reactions
Examples of Enzyme-Catalyzed
Reactions
Chymotrypsin is an enzyme that catalyzes
the hydrolysis of peptide bonds, withsome speciĂžcity for residues containing
aromatic side chains. Chymotrypsin also cleaves peptide bonds at other sites,
such as leucine, histidine, and glutamine, but with a lower frequency than at
aromatic amino acid residues. It also catalyzes the hydrolysis of ester bonds.
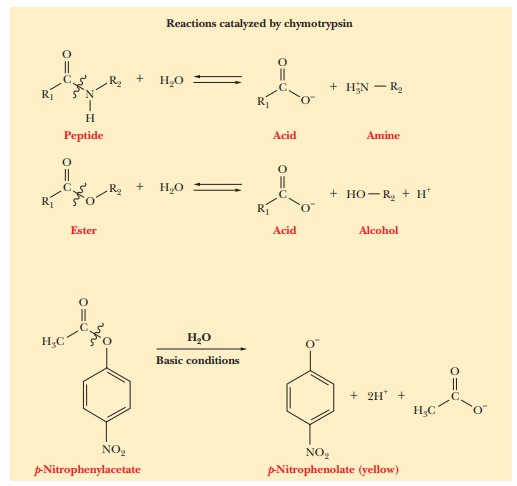
Although
ester hydrolysis is not important to the physiological role of chy-motrypsin in
the digestion of proteins, it is a convenient model system for investigating
the enzymeÕs catalysis of hydrolysis reactions. The usual labora-tory procedure
is to use p-nitrophenyl esters as the
substrate and to monitor the progress of the reaction by the appearance of a
yellow color in the reaction mixture caused by the production of p-nitrophenolate ion.
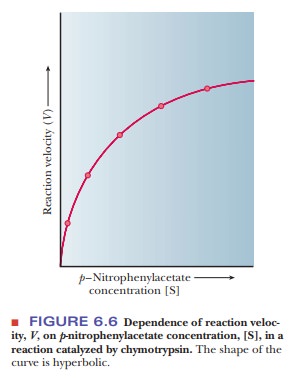
In a
typical reaction in which a p-nitrophenyl
ester is hydrolyzed by chymo-trypsin, the experimental rate of the reaction
depends on the concentration of the substrateÑin this case, the p-nitrophenyl ester. At low substrate
concen-trations, the rate of reaction increases as more substrate is added. At
higher substrate concentrations, the rate of the reaction changes very little
with the addition of more substrate, and a maximum rate is reached. When these
results are presented in a graph, the curve is hyperbolic (Figure 6.6).
Another
enzyme-catalyzed reaction is the one catalyzed by the enzyme aspar-tate transcarbamoylase (ATCase).
This reaction is the first step in a pathwayleading to the formation of
cytidine triphosphate (CTP) and uridine triphos-phate (UTP), which are
ultimately needed for the biosynthesis of RNA and DNA. In this reaction,
carbamoyl phosphate reacts with aspartate to produce carbamoyl aspartate and
phosphate ion.
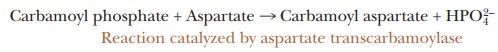
Reaction catalyzed by aspartate transcarbamoylase
The rate
of this reaction also depends on substrate concentrationÑin this case, the
concentration of aspartate (the carbamoyl phosphate concentration is kept
constant). Experimental results show that, once again, the rate of the reaction
depends on substrate concentration at low and moderate concentrations, and,
once again, a maximum rate is reached at high substrate concentrations.
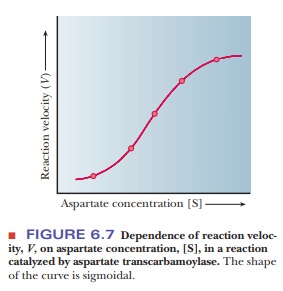
There
is, however, one very important difference. For this reaction, a graph showing
the dependence of reaction rate on substrate concentration has a sig-moidal
rather than hyperbolic shape (Figure 6.7).
Why do chymotrypsin and ATCase have different velocity curves?
The
results of experiments on the reaction kinetics of chymotrypsin and aspartate
transcarbamoylase are representative of experimental results obtained with many
enzymes. The overall kinetic behavior of many enzymes resembles that of
chymotrypsin, while other enzymes behave similarly to aspartate transcarbamoylase.
We can use this information to draw some general conclusions about the behavior
of enzymes. The comparison between the kinetic behaviors of chymotrypsin and
ATCase is reminiscent of the relationship between the oxygen-binding behaviors
of myoglobin and hemoglobin. ATCase and hemoglobin are allosteric proteins;
chymotrypsin and myoglobin are not. (Recall that allosteric proteins are the
ones in which subtle changes at one site affect structure and function at
another site. Cooperative effects, such as the fact that the binding of the
Ăžrst oxygen molecule to hemoglobin makes it easier for other oxygen molecules
to bind, are a hallmark of allosteric proteins.) The differences in behavior
between allosteric and nonallosteric proteins can be understood in terms of
models based on structural differences between the two kinds of proteins. When
we encounter the mechanisms of the many enzyme-catalyzed reactions in
subsequent, we shall need a model that explains the hyperbolic plot of kinetic
data for nonallosteric enzymes and another model that explains the sigmoidal
plot for allosteric enzymes. The Michaelis-Menten model is widely used for
nonallosteric enzymes, and several models are used for allosteric enzymes.
Summary
Chymotrypsin is an enzyme
that cleaves peptides near amino acids with aromatic side-chains. It can be
studied by using a substrate analog con-taining p-nitrophenylacetate.
When the velocity of chymotrypsin is plotted
versus its substrate, the curve is a hyperbola.
Aspartate transcarbamoylase is an enzyme that
is involved in the synthesis of nucleotides.
When the velocity of aspartate
transcarbamoylase is plotted versus aspar-tate, the curve is sigmoidal.
The
difference between the velocity curves for chymotrypsin and aspar-tate
transcarbamoylase demonstrates the difference between an allosteric enzyme and
a nonallosteric enzyme.
Related Topics