Chapter: Biochemistry: The Behavior of Proteins: Enzymes
Kinetics versus Thermodynamics
Kinetics versus Thermodynamics
The rate
of a reaction and its thermodynamic favorability are two different topics,
although they are closely related. This is true of all reactions, whether or
not a catalyst is involved. The difference between the energies of the
reactants (the initial state) and the energies of the products (the ├×nal state)
of a reaction gives the energy change for that reaction, expressed as the standard freeenergy change, or ŌłåG┬░. Energy
changes can be described by several relatedthermodynamic quantities. We shall
use standard free energy changes for our discussion; the question of whether a
reaction is favored depends on ŌłåG┬░. Enzymes, like all
catalysts, speed up reactions, but they cannot alter the equilibrium constant
or the free energy change. The reaction rate depends on the free energy of
activation or activation energy (ŌłåG┬░1), the
energy input required to initiate the reaction. The activation energy for an
uncatalyzed reaction is higher than that for a catalyzed reaction; in other
words, an uncatalyzed reaction requires more energy to get started. For this
reason, its rate is slower than that of a catalyzed reaction.
The
reaction of glucose and oxygen gas to produce carbon dioxide and water is an
example of a reaction that requires a number of enzymatic catalysts:
Glucose + 6O2 - > 6CO2 + 6H2O
This reaction
is thermodynamically favorable
(spontaneous in the thermodynamic sense) because its free energy change is
negative ( ŌłåG┬░ = -2880 kJ mol-1 = -689 kcal mol-1).
If a reaction is spontaneous, does that mean it will be fast?
Note
that the term spontaneous does not
mean ├Æinstantaneous.├ō Glucose is stable in air with an unlimited supply of
oxygen. The energy that must be supplied to start the reaction (which then
proceeds with a release of energy)Ñthe activation energyÑis conceptually
similar to the act of pushing an object to the top of a hill so that it can
then slide down the other side.
Activation
energy and its relationship to the free energy change of a reaction can best be
shown graphically. In Figure 6.1a, the x
coordinate shows the extent to which the reaction has taken place, and the y coordinate indicates free energy for
an idealized reaction. The activation
energy profile shows the interme- diate stages of a reaction, those between
the initial and final states. Activation energy profiles are essential in the
discussion of catalysts. The activation energy directly affects the rate of
reaction, and the presence of a catalyst speeds up a reaction by changing the
mechanism and thus lowering the activation energy. Figure 6.1a plots the
energies for an exergonic, spontaneous reaction, such as the complete oxidation
of glucose. At the maximum of the curve connect-ing the reactants and the
products lies the transition state
with the necessary amount of energy and the correct arrangement of atoms to
produce products. The activation energy can also be seen as the amount of free
energy required to bring the reactants to the transition state.

The
analogy of traveling over a mountain pass between two valleys is frequently
used in discussions of activation energy profiles. The change in energy
corresponds to the change in elevation, and the progress of the reaction cor-
responds to the distance traveled. The analogue of the transition state is the
top of the pass. Considerable effort has gone into elucidating the intermediate
stages in reactions of interest to chemists and biochemists and determining the
pathway or reaction mechanism that lies between the initial and final states.
Reaction dynamics, the study of the intermediate stages of reaction mecha-
nisms, is currently a very active field of research.
The most
important effect of a catalyst on a chemical reaction is appar-ent from a
comparison of the activation energy profiles of the same reaction, catalyzed
and uncatalyzed, as shown in Figure 6.1b. The standard free energy change for
the reaction, G┬Ī, remains unchanged
when a catalyst is added, but the activation energy, G┬Ī├Ā, is lowered. In the hill-and-valley analogy, the cata-lyst is a
guide that finds an easier path between the two valleys. A similar com-parison
can be made between two routes from San Francisco to Los Angeles. The highest
point on Interstate 5 is Tejon Pass (elevation 4400 feet) and is analogous to
the uncatalyzed path. The highest point on U.S. Highway 101 is not much over
1000 feet. Thus, Highway 101 is an easier route and is analogous to the
catalyzed pathway. The initial and final points of the trip are the same, but
the paths between them are different, as are the mechanisms of catalyzed and uncatalyzed reactions. The
presence of an enzyme lowers the activation energy needed for substrate
molecules to reach the transition state. The con-centration of the transition
state increases markedly. As a result, the rate of the catalyzed reaction is
much greater than the rate of the uncatalyzed reaction. Enzymatic catalysts
enhance a reaction rate by many powers of 10.
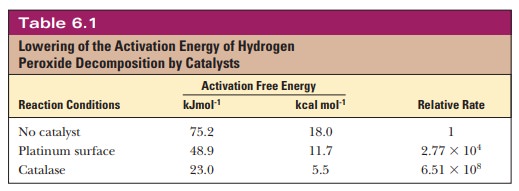
The biochemical reaction in which hydrogen
peroxide (H2O2) is converted to water and oxygen provides
an example of the effect of catalysts on activation energy.
2H2O2 - > 2H2O
+ O2
The activation energy of this reaction is lowered if the reaction is allowed to proceed on platinum surfaces, but it is lowered even more by the enzyme catalase. Table 6.1 summarizes the energies involved.
Will a reaction go faster if you raise the temperature?
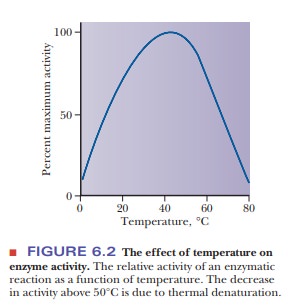
Raising
the temperature of a reaction mixture increases the energy available to with
temperature occurs only to a limited extent with biochemical reactions. It
reaction increases with temperature. One might be tempted to assume that this
is universally true for biochemical reactions. In fact, increase of reaction rate
is helpful to raise the temperature at ├×rst, but eventually there comes a point
at which heat denaturation of the enzyme is reached. Above this temperature,
adding more heat denatures more enzyme and slows down the reaction. Figure 6.2
shows a typical curve of temperature effect on an enzyme- catalyzed reaction.
The preceding Biochemical Connections box describes another way in which the
speci├×city of enzymes is of great use.
Summary
Thermodynamics of a biochemical reaction refers to whether a
reaction is spontaneous. A spontaneous reaction has a negative Gibbs free
energy or G┬Ī.
Kinetics refers to how fast a reaction occurs. A reaction may have
a nega- tive G┬Ī and still not happen quickly.
Enzymes speed up a reaction by lowering the activation energy of a
reac-tion. They help the substrate and enzyme attain the transition
state, the high point on an energy diagram for the reaction.
Related Topics