Chapter: Biochemistry: The Behavior of Proteins: Enzymes
Enzyme-Substrate Binding
Enzyme–Substrate Binding
In an
enzyme-catalyzed reaction, the enzyme binds to the substrate (one of the reactants) to form a complex. The formation
of the complex leads to the formation of the transition-state species, which
then forms the product. The nature of transition states in enzymatic reactions
is a large Ăželd of research in itself, but some general statements can be made
on the subject. A substrate binds, usually by noncovalent interactions, to a
small portion of the enzyme called the active
site, frequently situated in a cleft or crevice in the protein and
consistingof certain amino acids that are essential for enzymatic activity
(Figure 6.3). The catalyzed reaction takes place at the active site, usually in
several steps.
Why do enzymes bind to substrates?
The Ăžrst
step is the binding of substrate to the enzyme, which occurs because of highly
speciĂžc interactions between the substrate and the side chains and backbone
groups of the amino acids making up the active site. Two important models have
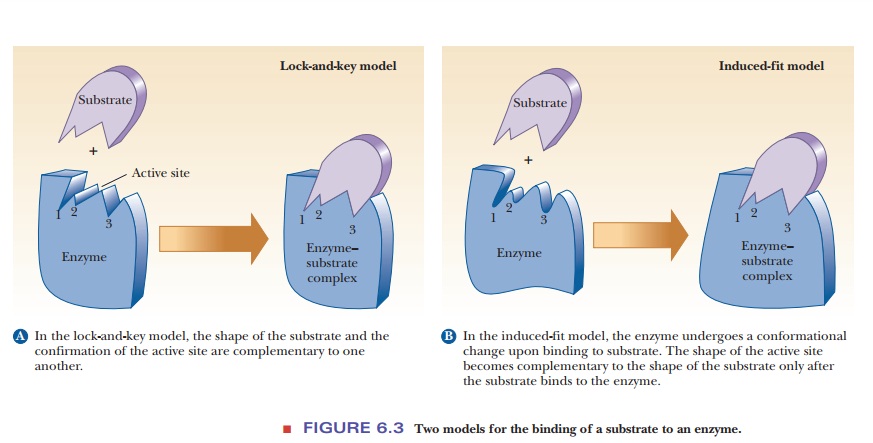
been developed to describe the binding process. The Ăžrst, the lock-and-key model, assumes a high
degree of similarity between the shape of the substrate and the geometry of the
binding site on the enzyme (Figure 6.3a). The substrate binds to a site whose
shape complements its own, like a key in a lock or the correct piece in a
three-dimensional jigsaw puzzle. This model has intuitive appeal but is now
largely
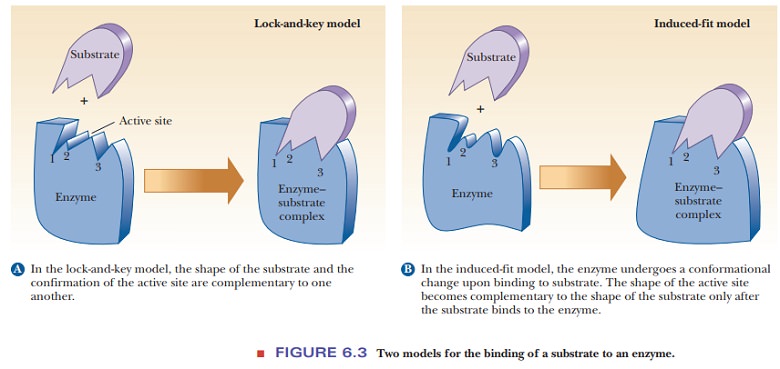
The second model takes
into account the fact that proteins have some three-dimensional Ăźexibility.
According to this induced-fit model,
the binding of the substrate induces a conformational change in the enzyme that
results in a complementary Ăžt after the substrate is bound (Figure 6.3b). The
binding site has a different three-dimensional shape before the substrate is
bound. The induced-Ăžt model is also more attractive when we consider the nature
of the transition state and the lowered activation energy that occurs with an
enzyme-catalyzed reaction. The enzyme and substrate must bind to form the ES
complex before anything else can happen. What would happen if this binding were
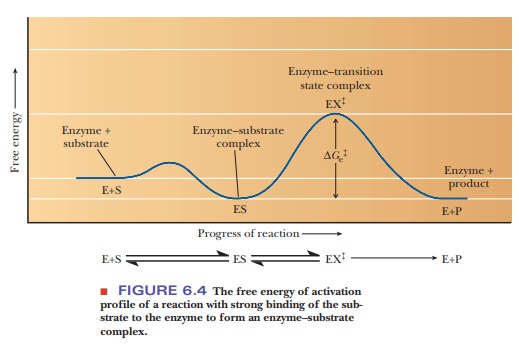
too perfect? Figure 6.4 shows what happens when E and S bind. An attraction
must exist between E and S for them to bind. This attraction causes the ES
complex to be lower on an energy diagram than the E + S at the start. Then the
bound ES must attain the conformation of the transition state EXĂ . If the
binding of E and S to form ES were a perfect Ăžt, the ES would be at such a low
energy that the difference between ES and EXĂ would
be very large. This would slow down the rate of reaction. Many studies have
shown that enzymes increase the rate of reaction by lowering the energy of the
transition state, EXĂ , while raising the energy of
the ES complex. The induced-Ăžt model certainly supports this last consideration
better than the lock-and-key model; in fact, the induced-Ăžt model mimics the
transition state.
After
the substrate is bound and the transition state is subsequently formed,
catalysis can occur. This means that bonds must be rearranged. In the
tran-sition state, the substrate is bound close to atoms with which it is to
react. Furthermore, the substrate is placed in the correct orientation with respect
to those atoms. Both effects, proximity and orientation, speed up the reaction.
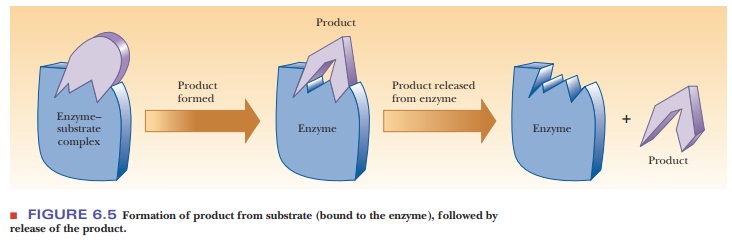
As bonds are broken and new bonds are formed, the substrate is transformed into
product. The product is released from the enzyme, which can then catalyze the
reaction of more substrate to form more product (Figure 6.5).
Each enzyme has its
own unique mode of catalysis, which is not surprising in view of enzymesÕ great
specificity. Even so, some general modes of catalysis exist in enzymatic
reactions. Two enzymes, chymotrypsin and aspartate transcarbamoylase, are good
examples of these general principles.
Summary
Before a reaction can be catalyzed, the enzyme
and substrate must bind.The substrate binds to the enzyme in a special pocket
called the active site.
Binding
to the active site is reversible and occurs through noncovalent interactions.
Two
models are often used to describe the binding: the lock-and-key model and the
induced-fit model.
The induced-fit model is the more accurate
description of formation of the ES complex, as it explains how the binding of E
+ S leads toward establishment of the transition state.
Related Topics