Solution, Application, GrahamŌĆÖs Law - Mixture of gases - DaltonŌĆÖs law of partial pressures | 11th Chemistry : UNIT 6 : Gaseous State
Chapter: 11th Chemistry : UNIT 6 : Gaseous State
Mixture of gases - DaltonŌĆÖs law of partial pressures
Mixture
of gases - DaltonŌĆÖs law of partial pressures
Studies of non-reacting gaseous mixtures showed that in a
gaseous mixture each component behaves independently. For a gaseous mixture, it
is important to know, how the pressure of individual component contributes to
the total pressure of the mixture.
John Dalton stated that "the total pressure of a
mixture of non-reacting gases is the sum of partial pressures of the gases
present in the mixture" where the partial pressure of a component gas is
the pressure that it would exert if it were present alone in the same volume
and temperature. This is known as DaltonŌĆÖs
law of partial pressures.
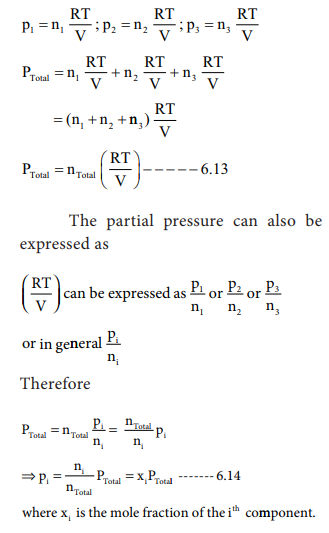
i.e., for a mixture containing three gases 1, 2 and 3 with partial pressures
p1, p2 and p3 in a container with volume V,
the total pressure Ptotal will be give by
Ptotal = p1 + p2 + p3
------- (6.12)
Assuming that the gases behave ideally,
Application
of DaltonŌĆÖs law
In a reaction involving the collection of gas by downward
displacement of water, the pressure of dry vapor collected can be calculated
using DaltonŌĆÖs law.
Pdry gas collected = ptotal - pwater vapour
pwater vapour is generally referred as aqueous
tension and its values are available
for air at various temperatures.
Let us understand Dalton's law by solving this problem. A
mixture of gases contains 4.76 mole of Ne, 0.74 mole of Ar and 2.5 mole of Xe.
Calculate the partial pressure of gases, if the total pressure is 2 atm. at a fixed temperature.
Solution:
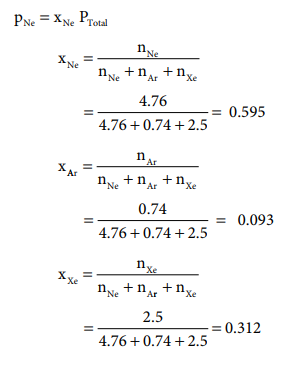
PNe = xNe PTotal = 0.595 ├Ś 2
= 1.19 atm.
PAr = xAr PTotal = 0.093 ├Ś 2
= 0.186 atm.
PXe = xXe PTotal = 0.312 ├Ś 2
= 0.624 atm.
GrahamŌĆÖ s Law of Diffusion
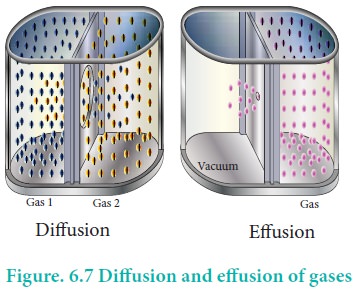
Gases have a tendency to occupy all the available space.
When two non -reactive gases are allowed to mix, the gas molecules migrate from
region of higher concentration to a region of lower concentration. This
property of gas which involves the movement of the gas molecules through
another gases is called diffusion. Effusion is another process in which a gas
escapes from a container through a very small hole. The rate of diffusion or
effusion is inversely proportional to the square root of molar mass. This
statement is called Graham's law of diffusion/effusion.
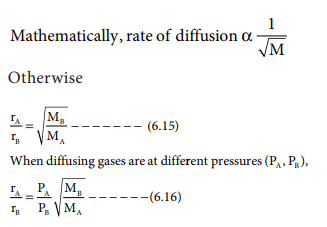
where rA and rB are the rates of
diffusion of A and B and the MA and MB are their
respective molar masses.
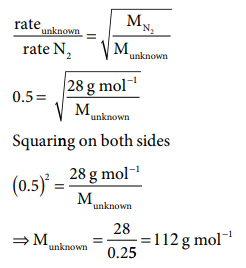
1. An unknown gas diffuses at a rate of 0.5 time that of
nitrogen at the same temperature and
pressure. Calculate the molar mass of the unknown gas
Solution:
Related Topics