Solved Example Problems with Answer - Evaluate Yourself: Gaseous State (Chemistry) | 11th Chemistry : UNIT 6 : Gaseous State
Chapter: 11th Chemistry : UNIT 6 : Gaseous State
Evaluate Yourself: Gaseous State (Chemistry)
Evaluate Yourself
1. Freon-12, the compound widely
used in the refrigerator system as coolant causes depletion of ozone layer. Now
it has been replaced by eco-friendly compounds. Consider 1.5 dm3
sample of gaseous Freon at a pressure of 0.3 atm. If the pressure is changed to 1.2 atm. at a constant temperature, what will be the volume of the gas
increased or decreased?
Answer:
Volume of freon (V1) =
1.5 dm3
Pressure (P1) = 0.3
atm
'T' isconstant
P2= 1.2 atm
V2= ?
Ōł┤ P1V1 = P2V2
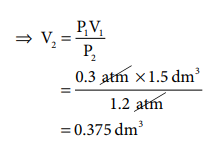
=0.375 dm3
Ōł┤ Volume decreased from 1.5 dm3 to 0.375
dm3
2. Inside a certain automobile
engine, the volume of air in a cylinder is 0.375 dm3, when the
pressure is 1.05 atm. When the gas is
compressed to a volume of 0.125 dm3
at the same temperature, what is the pressure of the compressed air?
Answer:
V1 = 0.375 dm3
V2 = 0.125
P1 = 1.05 atm
P2 = ?
'T' -
Constant
P1V1 = P2V2
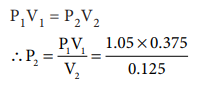
P2 = 3.8 dm3
3. A sample of gas has a volume of
3.8 dm3 at an unknown temperature. When the sample is submerged in
ice water at 0 ┬░C, its volume gets reduced to 2.27 dm3. What is its
initial temperature?
Answer:
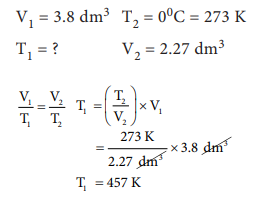
4. An athlete in a kinesiology
research study has his lung volume of 7.05 dm3 during a deep
inhalation. At this volume the lungs contain 0.312 mole of air. During
exhalation the volume of his lung decreases to 2.35 dm3. How many
moles of air does the athlete exhale during exhalation? (assume pressure and
temperature remain constant)
Answer:
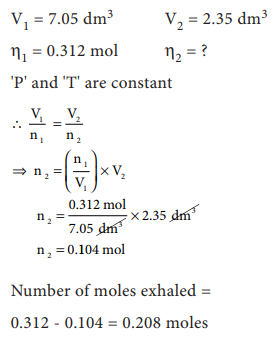
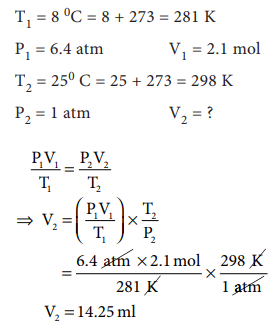
5. A small bubble rises from the
bottom of a lake where the temperature and pressure are 8┬░ C and 6.4 atm. to the water surface, where the
temperature is 25┬░C and pressure is 1 atm.
Calculate the final volume in (mL) of the bubble, if its initial volume is 2.1
mL.
Answer:
6. (a) A mixture of He and O2 were used in the ŌĆśairŌĆÖ tanks of underwater divers for deep dives. For a particular dive 12 dm3 of O2 at 298 K, 1 atm. and 46 dm3 of He, at 298 K, 1 atm. were both pumped into a 5 dm3 tank. Calculate the partial pressure of each gas and the total pressure in the tank at 298 K (b) A sample of solid KClO3 (potassium chlorate) was heated in a test tube to obtain O2 according to the reaction
2KClO3ŌåÆ 2KCl + 3O2
(b) The oxygen gas was collected by
downward displacement of water at 295 K. The total pressure of the mixture is
772 mm of Hg. The vapour pressure of water is 26.7 mm of Hg at 300K. What is
the partial pressure of the oxygen gas?
Answer:

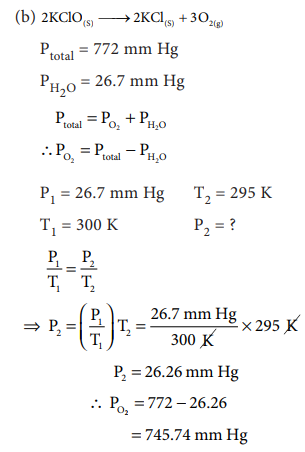
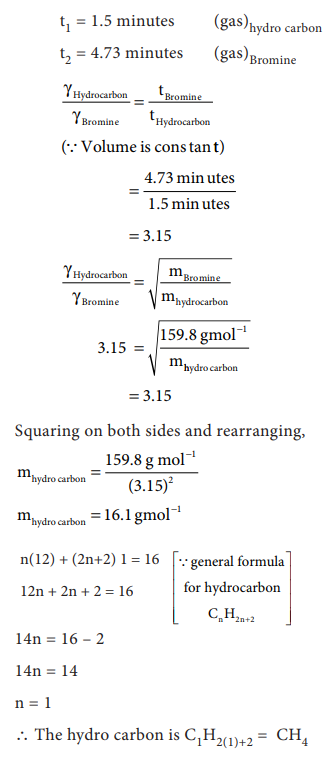
7. A flammable hydrocarbon gas of
particular volume is found to diffuse through a small hole in 1.5 minutes.
Under the same conditions of temperature and pressure an equal volume of
bromine vapour takes 4.73 min to diffuse through the same hole. Calculate the
molar mass of the unknown gas and suggest what this gas might be, (Given that
molar mass of bromine = 159.8 g/mole)
Answer:
8. Critical temperature of H2O,
NH3, and CO2 are 647.4, 405.5 and 304.2 K, respectively.
When we start cooling from a temperature of 700 K which will liquefy first and
which will liquefy finally?
Answer:
Critical temperature of a gas is
defined as the temperature above which it cannot be Ōł┤
liquified
even at high pressures. When cooling starts from 700 K, H2O will liquify
first, then followed by ammonia and finally carbon dioxide will
liquify.
Related Topics