Chapter: 11th Chemistry : UNIT 6 : Gaseous State
Graham’ s Law of Diffusion
Graham’ s Law of Diffusion
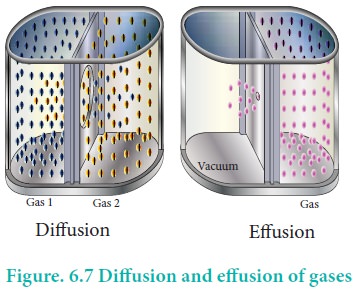
Gases have a tendency to occupy all the available space. When two non -reactive gases are allowed to mix, the gas molecules migrate from region of higher concentration to a region of lower concentration. This property of gas which involves the movement of the gas molecules through another gases is called diffusion. Effusion is another process in which a gas escapes from a container through a very small hole. The rate of diffusion or effusion is inversely proportional to the square root of molar mass. This statement is called Graham's law of diffusion/effusion.
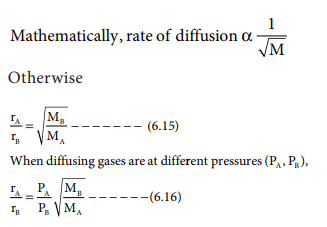
where rA and rB are the rates of diffusion of A and B and the MA and MB are their respective molar masses.
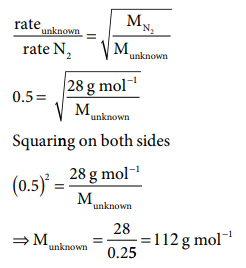
1. An unknown gas diffuses at a rate of 0.5 time that of nitrogen at the same temperature and pressure. Calculate the molar mass of the unknown gas
Solution:
Related Topics