Chapter: 11th Chemistry : UNIT 6 : Gaseous State
Deviation from ideal gas behaviour
Deviation
from ideal gas behaviour
The kinetic theory of gases which is the basis for the gas
equation (PV=nRT), assumes that the individual gas molecules occupy negligible
volume when compared to the total volume of the gas and there is no attractive
force between the gas molecules. Gases whose behaviour is consistent with these
assumptions under all conditions are called ideal gases. But in practice both
these assumptions are not valid under all conditions. For example, the fact
that gases can be liquefied shows that the attractive force exists among
molecules. Hence, there is no gas which behaves ideally under all conditions.
The non-ideal gases are called real gases. The real gases tend to approach the
ideal behaviour under certain conditions.
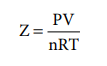
Compressibility factor Z
The deviation of real gases from ideal behaviour is
measured in terms of a ratio of PV to nRT. This is termed as compressibility
factor. Mathematically,
For ideal gases PV = nRT, hence the compressibility
factor, Z = 1 at all temperatures and pressures. For these gases the plot of Z
vs P should be a straight line parallel to the pressure axis. When a gas
deviates from ideal behaviour, its Z value deviates from unity. For all gases,
at very low pressures and very high temperature the compressibility factor
approaches unity and they tend to behave ideally. The plot of the
compressibility factor vs pressure for some common gases are shown in Figure
6.8.

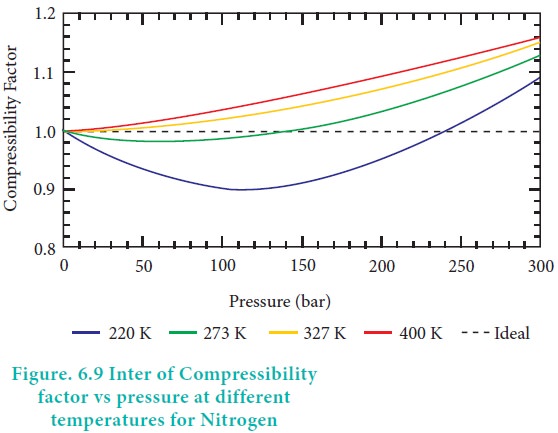
When the pressure is low, the volume of the container is
very large compared to the volume of the gas molecules so that individual
volume of the gas molecules can be neglected. In addition, the molecule in a
gas are far apart and attractive forces are negligible. As the pressure
increases, the density of gas also increases and the molecules are much closer
to one another. Hence, the intermolecular force becomes significant enough to
affect the motion of the molecules and the gas will not behave ideally.
At high temperatures the average kinetic energy of the
molecules is very high and hence inter moleclular attractions will become
insignificant. As the temperature decreases, the average kinetic energy of
molecules also decreases, hence the molecular attraction is enhanced. The
temperature at which a real gas obeys ideal gas law over an appreciable range
of pressure is called Boyle temperature or Boyle point. The Boyle point varies
with the nature of the gas. Above the Boyle point, for real gases, Z > 1,
ie., the real gases show positive deviation. Below the Boyle point, the real
gases first show a decrease for Z, reaches a minimum and then increases with
the increase in pressure. So, it is clear that at low pressure and at high
temperature, the real gases behave as ideal gases.
Compressibility factor for real gases
The compressibility factor Z for real gases can be
rewritten

Where Vreal is the molar volume of the real gas
and Videal is the molar volume of it when it behaves ideally.
Van der Waals Equation
J. D. Van der Waals made the first mathematical analysis
of real gases. His treatment provides us an interpretation of real gas
behaviour at the molecular level. He modified the ideal gas equation PV = nRT
by introducing two correction factors, namely, pressure correction and volume
correction.
Pressure Correction:
The pressure of a gas is directly proportional to the
force created by the bombardment of molecules on the walls of the container.
The speed of a molecule moving towards the wall of the container is reduced by
the attractive forces exerted by its neighbours. Hence, the measured gas
pressure is lower than the ideal pressure of the gas. Hence, van der Waals
introduced a correction term to this effect.
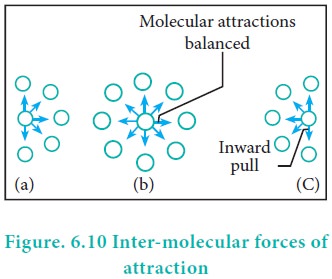
Van der Waals found out the forces of attraction
experienced by a molecule near the wall are directly proportional to the square
of the density of the gas.
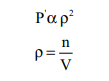
where n is the number of moles of gas and V is the volume
of the container
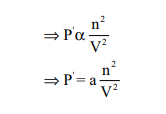
where a is proportionality constant and depends on the
nature of gas
Therefore,
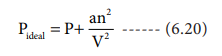
Volume Correction
As every individual molecule of a gas occupies a certain
volume, the actual volume is less than the volume of the container, V. Van der
Waals introduced a correction factor V' to this effect. Let us calculate the
correction term by considering gas molecules as spheres.
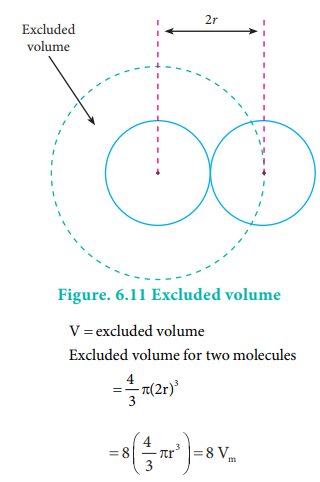
where vm
is a volume of a single molecule Excludeed volume for single molecule
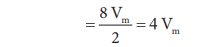
Excluded volume for n molecule = n(4Vm) = nb
Where b is van der waals constant whch is equal to 4 Vm
V' = nb
Videal = V - nb
.... (6.21)
Replacing the corrected pressure and volume in the ideal
gas equation PV=nRT we get the van der Waals equation of state for real gases
as below,
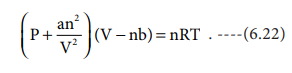
The constants a
and b are van der Waals constants and
their values vary with the nature of the gas. It is an approximate formula for
the non-ideal gas.
Related Topics