Chapter: 11th Chemistry : UNIT 6 : Gaseous State
Brief Questions and Answers: Gaseous State (Chemistry)
Gaseous State (Chemistry)
Brief Questions and Answers
26. State Boyle's law.
At
a given temperature, the volume occupied by a fixed mass of the gas is
inversely proportional to its pressure
Boyle's
law can be written as, V ŌłØ 1 / P
(or) V = K (1/P)
(or) PV = K
Q. A balloon filled with air at room temperature and cooled to a much lower temperature can be used as a model for CharleŌĆÖs law
27. Name two items that can serve as a model for Gay LusaacŌĆÖ law and explain.
1)
Candle experiment (Air contains 1/5 of oxygen experiment)
2)
Boiled Egg in a bottle experiment
28. Give the mathematical expression that relates gas volume and moles. Describe in words what the mathematical expression means.
ŌŚÅ
The mathematical expression that relates gas volume and moles is V ŌłØ n
ŌŚÅ
This mathematical expression is Avagadro hypothesis.(Avagadro Law). It states
that, equal volume of all gases under the same conditions of temperature and
pressure contains equal number of molecules.
29. What are ideal gases? In what way real gases differ from ideal gases.
ŌŚÅ
A gas which obeys the general gas equation (PV = nRT) under all conditions of
temperature and pressure is called an ideal gas.
ŌŚÅ
The real gases differ from ideal gas only at high temperature and low pressure.
30. Can a Van der Waals gas with a=0 be liquefied? explain.
ŌŚÅ
Generally 'a' value is a measure of magnitude of the intermolecular force of
attraction
ŌŚÅ
Since, a = 0 means, there is no intermolecular force of attraction exist
between the gas molecules.
ŌŚÅ
So, Vander Waal gas with a = 0 cannot be liquefied.
31. Suppose there is a tiny sticky area on the wall of a container of gas. Molecules hitting this area stick there permanently. Is the pressure greater or less than on the ordinary area of walls?
ŌŚÅ
The pressure of the gas will be greater than on the ordinary area of walls.
ŌŚÅ
As per the postulates of kinetic theory of gases, pressure exerted by the gas
is due to collision of its molecules on the wall of the container.
32. Explain the following observations
a) Aerated water bottles are kept under water during summer
b) Liquid ammonia bottle is cooled before opening the seal
c) The tyre of an automobile is inflated to slightly lesser pressure in summer than in winter
d) The size of a weather balloon becomes larger and larger as it ascends up into larger altitude
a)
Aerated water bottles are kept under water during summer:
ŌŚÅ
Aerated water contains air/CO2 dissolved in water (H2O).
ŌŚÅ
The solubility of gas decreases with rise in temperature. (Henry's Law)
ŌŚÅ
Thus, amount of free gas in the bottle increases in summer, which may result in
increase in pressure and ultimately explosion.
ŌŚÅ
To aviod this, they are kept under cold water.
b)
Liquid ammonia bottle is cooled before opening the seal:
ŌŚÅ
At room temperature, the vapour pressure of liquid ammonia is very high.
ŌŚÅ
On cooling, the vapour pressure decreases (Gay Lussac's Law applies)
ŌŚÅ
Therefore, the liquid ammonia will not splash out of the bottle.
c)
The tyre of an automobile is inflated to slightly lesser pressure in summer
than in winter:
ŌŚÅ
In summer, due to high temperature, the average kinetic energy of air molecules
inside the tube increases.
ŌŚÅ
Hence, the pressure on wall of the tube increases leads to burst of tyre.
ŌŚÅ
To aviod burst of tyre, it is better to have lesser pressure while inflate air
in summer than in winter.
d)
The size of a weather balloon becomes larger and larger as it ascends up into
larger altitude:
ŌŚÅ
As we go to higher altitude, the atmospheric pressure decreases.
ŌŚÅ
Thus, the pressure outside the balloon decreases.
ŌŚÅ
To maintain equilibrium with external pressure, the gas inside expands to
decrease its pressure. Hence, the size of the balloon increases.
33. Give suitable explanation for the following facts about gases.
a) Gases donŌĆÖt settle at the bottom of a container
b) Gases diffuse through all the space available to them
a)
Gases don't settle at the bottom of a container:
ŌŚÅ
Gases are very less denser and also negligible intermolecular force of
attraction between the particles of the gas. So, they are all free to move
separately.
b)
Gases diffuse through all the space available to them:
ŌŚÅ
The molecules in gases are far apart from each other due to negligible force of
attraction between them.
ŌŚÅ
Hence, they have greater kinetic energy.
ŌŚÅ
So, particles move very fast and colloide with one another causing them to
diffuse until they are evenly distributed throughout available space.
34. Suggest why there is no hydrogen (H2) in our atmosphere. Why does the moon have no atmosphere?
ŌŚÅ
Hydrogen is the lightest gas in the atmosphere. So, it rises up than the other
gases which are heavier. More over hydrogen highly reactive towards oxygen. So,
a major portion of hydrogen reacts and very less amount of it is present in the
upper level of the atmosphere.
ŌŚÅ
Moon has no atmosphere because ŌĆśg' value is small. The molecules of the
atmospheric gases on surface of moon have greater thermal velocity (escape
velocity than moon). That is why all the gaseous molecules have escaped and there
is no atmosphere.
35. Explain whether a gas approaches ideal behavior or deviates from ideal behaviour if
a) it is compressed to a smaller volume at constant temperature.
b) the temperature is raised at while keeping the volume constant
c) more gas is introduced into the same volume and at the same temperature
a)
It is compressed to a smaller volume at constant temperature.
Gases
deviates from ideal behavior because each molecule occupies definite volume.
b)
The temperature is raised at while keeping the volume constant
As
temperature is raised, kinetic energy of the molecule is greater. Hence, gas
approaches ideal behavior.
c)
More gas is introduced into the same volume and at the same temperature
More
gas is introduced into the container, there is no room for the molecules to
move around. So, each molecule occupies a definite volume. Hence, gas deviates
from ideal behaviour.
36. Which of the following gases would you expect to deviate from ideal behaviour under conditions of low temperature F2, Cl2, or Br2? Explain.
Bromine
deviates from ideal behaviour at low temperature. Because, molar mass of
bromine is greater than chlorine and fluorine. So, kinetic energy of the
molecules is less. Hence, it deviates from ideal behaviour.
37. Distinguish between diffusion and effusion.
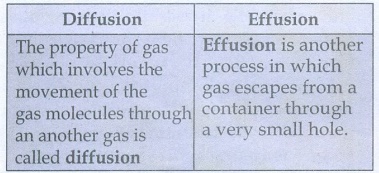
Diffusion
The property of gas which
involves the movement of the gas molecules through an another gas is called
diffusion
Effusion
Effusion is
another process in which gas escapes from a container through a very small
hole.
38. Aerosol cans carry clear warning of heating of the can. Why?
Aersol
can carry clear warning of heat of the can because of the high pressure that
can develop upon heating. Hence, can will burst (or) explode if there is any
residual hydrocarbon (or) if there is a naked flame nearby.
Q. When the driver of an automobile applies brake, the passengers are pushed toward the front of the car but a helium balloon is pushed toward back of the car. Upon forward acceleration the passengers are pushed toward the front of the car. Why?
39. Would it be easier to drink water with a straw on the top of Mount Everest?
At
the top of Mount Everest, air pressure is low. Hence, water is not pushed up
the straw through your mouth. So, it is not easier to drink water.
40. Write the Van der Waals equation for a real gas. Explain the correction term for pressure and volume
The
Vander Waals equation for real gas is
(P
+ [an2/V2] ) (V ŌĆō nb) = nRT
Pressure
Correction: For an ideal gas equation, PidealVideal
= nRT. But for a real gas the measured pressure is smaller than Pideal
due to attractive forces between real gas molecules. Therefore to make the
pressure equivalent to Pideal . We have to add some correction term
to the measured pressure. The quantity
an2
/ V2 is called Pressure Correction
Volume
Correction: For an ideal gas Pideal Videal
= nRT. But for a real gas the measured volume is larger than Videal
because real gas molecule occupies a definite volume. Therefore, to make the
volume equivalent Videal some correction term must be subtracted
from the measured volume. The quantity nb is called Volume Correction
41. Derive the values of critical constants in terms of vander waals constants. [or] Derive the values of van der Waals equation constants in terms of critical constants.
The van der Waals equation for n
moles is
(P + [an2/V2]) (V ŌĆō nb)
= nRT ............. (1)
For 1 mole
(P + [a/V2]) (V ŌĆō b) =
RT .............
(2)
From the equation we can derive
the values of critical constants Pc, Vc and Tc in terms of a and b, the van der
Waals constants, On expanding the above equation
PV+ a/V ŌłÆ Pb ŌłÆ ab/V2 ŌłÆ
RT = 0 .... ...(3)
Multiply equation (3) by V2
/ P
V2/P
(PV + a/V ŌłÆ Pb ŌĆō ab/V2 ŌĆō RT ) = 0
V3
+ aV/P ŌĆō bV2 ŌĆō ab/P ŌĆō RTV2/P = 0
ŌĆ”ŌĆ”..(4)
When
the above equation is rearranged in powers of V
V3
+ ([RT / P ] + b)V2 + [a / p ]V ŌĆō [ab / P ] = 0 ŌĆ”ŌĆ”..(5)
The
equation (5) is a cubic equation in V. On solving this equation, we will get
three solutions.
At
the critical point all these three solutions of V are equal to the critical
volume Vc. The pressure and temperature becomes Pc and Tc respectively
i.e.,
V = VC
V
ŌłÆ VC = 0
(V
ŌłÆ VC)3 = 0
V3
ŌłÆ 3VCV2 + 3VC2V ŌłÆ VC3
= 0 ŌĆ”ŌĆ”ŌĆ”..(6)
As
equation (5) is identical with equation (6), we can equate the coefficients of
V2 , V and constant terms in (5) and (6).
ŌłÆ3VcV2
= [ RTc/Pc + b ] V2
3Vc
= RTc/Pc + b ŌĆ”ŌĆ”ŌĆ”ŌĆ”ŌĆ” (7)
3Vc2
= a / Vc ŌĆ”ŌĆ”ŌĆ”ŌĆ”ŌĆ” (8)
Vc3
= ab / Pc ŌĆ”ŌĆ”ŌĆ”..(9)
Divide
equation (9) by equation (8)
Vc
/ 3V = ab/Pc / a/P
Vc3
/ 3 = b
i.e.
V c = 3b ŌĆ”ŌĆ”ŌĆ”ŌĆ”ŌĆ”ŌĆ”. (10)
when
equation (10) is substituted in (8)
3Vc2
= a/Pc
Pc
= a/3Vc2 = a / 3(3b2) = a / 3├Ś9b2 =
a / 27b2
Pc
= a / 27b2 ŌĆ”ŌĆ”ŌĆ”ŌĆ” (11)
Substituting
the values of Vc and Pc, in equation (7),
3VC
= b + [ RTC / PC ]
3(3b)
= b + [ RTC 27b2 / a]
RTC
27b2 / a = 9b ŌłÆ b
TC
= 8ba / 27Rb2
TC
= 8a / 27Rb ŌĆ”ŌĆ”ŌĆ”ŌĆ”ŌĆ”. (12)
The
critical constants can be calculated using the values of van der waals constant
of a gas and vice versa.
a
= 3 V2C PC ;
b
= Vc / 3
42. Why do astronauts have to wear protective suits when they are on the surface of moon?
On
the surface of moon, there is no oxygen to breathe, no air pressure, extremely
cold and filled with dangerous radiation. To avoid these difficulty, astronauts
have to wear protective suit.
43. When ammonia combines with HCl, NH4Cl is formed as white dense fumes. Why do more fumes appear near HCl?
Ammonia
diffuses faster than HCl due its
lower molar mass. (According to Graham Law of diffusion) That why more fumes
near HCl.
44. A sample of gas at 15 ┬░C at 1 atm. has a volume of 2.58 dm3. When the temperature is raised to 38 ┬░C at 1 atm does the volume of the gas increase? If so, calculate the final volume.
Answer:
T1
= 15┬░C + 273
T2
= 38 + 273
T1
= 288 K
T2
= 311 K
V1
= 2.58 dm3
V2
= ?
(P = 1 atm constant)
Solution:
V1
/ T1 = V2 / T2
V2
= V1/T1 ├Ś T2 = [2.58 dm3 /
288 K ] ├Ś 311 K
V2
= 2.78 dm3
ie.,
volume increased from 2.58 dm3 to 2.78 dm3
45. A sample of gas has a volume of 8.5 dm3 at an unknown temperature. When the sample is submerged in ice water at 0 ┬░C, its volume gets reduced to 6.37 dm3. What is its initial temperature?
Answer:
Given
V1
= 8.5 dm3
V2
= 6.37 dm3
T1
= ?
T2
= 0┬░
C
= 273 K
Solution:
V1
/ T1 = V2 / T2
V1
├Ś (T2 / V2) = T1
=
T1
ŌćÆ 8.5 dm3 ├Ś ( 273 K /
6.37 dm3 )
ŌćÆ T1 = 364.28 K
46. Of two samples of nitrogen gas, sample A contains 1.5 moles of nitrogen in a vessel of volume of 37.6 dm3 at 298K, and the sample B is in a vessel of volume 16.5 dm3 at 298K. Calculate the number of moles in sample B.
Answer:
Given
nA
= 1.5 mol
nB
= ?
VA
= 37.6 dm3
VB
= 16.5 dm3
(T
= 298K constant)
Solution
:
VA
/ nA = VB / nB
nB
= (nA / VA) VB
=
(1.5 mol / 37.6 dm3 ) ├Ś 16.5 dm3 = 0.66 mol
47. Sulphur hexafluoride is a colourless, odourless gas; calculate the pressure exerted by 1.82 moles of the gas in a steel vessel of volume 5.43 dm3 at 69.5 ┬░C, assuming ideal gas behaviour
Answer:
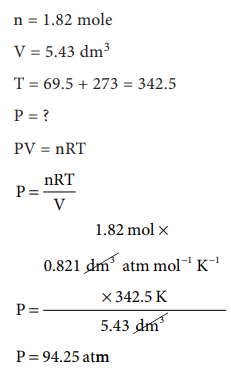
[Alternative
Answer] Answers are in doubt
Given:
n
= 1.82 mole
V
= 5.43 dm3
T
= 69.5 + 273 = 342.5
P
= ?
PV
= nRT
Solution:
P
= nRT / V
P
= [1.82 ├Ś 0.0821 ├Ś 342.5] / 5.43
P
= 9.425 atm
48. Argon is an inert gas used in light bulbs to retard the vaporization of the tungsten filament. A certain light bulb containing argon at 1.2 atm and 18┬░C is heated to 85┬░C at constant volume. Calculate its final pressure in atm.
Answer:
Given:
P1
= 1.2 atm
T1
= 18┬░C + 273 = 291K
T2
= 85┬░C + 273 = 358 K
P2
= ?
Solution:
P1
/ T1 = P2 / T2
P2
= (P1/T1 ) ├Ś T2
=
(1.2 atm / 291K) ├Ś 358 K
P2
= 1.48 atm
49. A small bubble rises from the bottom of a lake where the temperature and pressure are 6┬░C and 4 atm. to the water surface, where the temperature is 25┬░C and pressure is 1 atm. Calculate the final volume in (mL) of the bubble, if its initial volume is 1.5 mL.
Answer:
Solution:
T1
= 6┬░C + 273 = 279 K
P1
= 4 atm
V1
= 1.5 ml
T2
= 25┬░ C + 273 = 298 K
P2
= 1 atm
V2
= ?
P1V1
/ T1 = P2V2 / T2
V2
= [ P1V1 / T1 ] ├Ś [T2 / P2]
=
[ 4 atm ├Ś 1.5 ml ├Ś 298 K ] / [ 279 K ├Ś 1 atm ]
V2
= 6.41 mol
50. Hydrochloric acid is treated with a metal to produce hydrogen gas. Suppose a student carries out this reaction and collects a volume of 154.4 ├Ś 10-3 dm3 of a gas at a pressure of 742 mm of Hg at a temperature of 298 K. What mass of hydrogen gas (in mg) did the student collect?
Answer:
Given:
V
= 154.4 ├Ś 10ŌłÆ3 dm3,
P
= 742 mm of Hg
T
= 298 K
m
= ?
n
= PV / RT
=
[ 742 mm Hg ├Ś 154.4 ├Ś 10ŌłÆ3L ] / [ 62 mm Hg LKŌłÆ1mol ŌłÆ1
├Ś 298 K ]
=
0.006 mol
n
= mass / Molar mass
Mass
= n ├Ś Molar mass
=
0.006 ├Ś 2.016
=
0.0121g = 12.1 mg
51. It takes 192 sec for an unknown gas to diffuse through a porous wall and 84 sec for N2 gas to effuse at the same temperature and pressure. What is the molar mass of the unknown gas?
Answer:
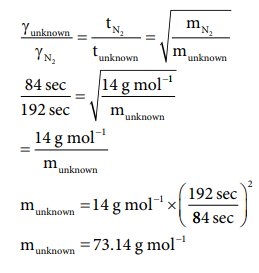
[Answers are in doubt ]
(84
/ 192)2 = 28 g molŌłÆ1 / munknown
munknown
= 28g molŌłÆ1 ├Ś (192 sec / 84 sec )2
munknown
= 146.28 molŌłÆ1
52. A tank contains a mixture of 52.5 g of oxygen and 65.1 g of CO2 at 300 K the total pressure in the tanks is 9.21 atm. Calculate the partial pressure (in atm.) of each gas in the mixture.
Answer:
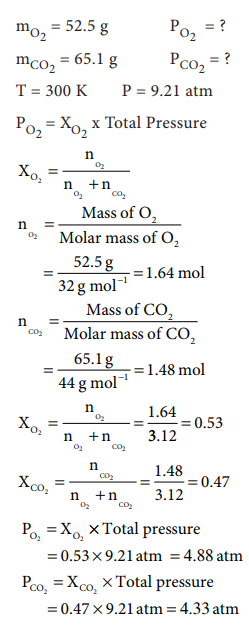
54. A combustible gas is stored in a metal tank at a pressure of 2.98 atm at 25 ┬░C. The tank can withstand a maximum pressure of 12 atm after which it will explode. The building in which the tank has been stored catches fire. Now predict whether the tank will blow up first or start melting? (Melting point of the metal = 1100 K).
Answer:
Pressure of the gas in the tank at its melting point
T1
= 298K;
P1
= 2.98 atm ;
T2
= 1100 K;
P2
= ?
Solution:
P1/T1
= P2/T2
ŌćÆ P2 = P1 ├Ś (T2
/ T1)
=
(2.98 atm ├Ś 1100 K ) / 298 K = 11 atm
At 1100 K the pressure of the gas inside the tank will become 11 atm. Given that tank can withstand a maximum pressure of 12 atm, the tank will start melting first.
Related Topics