Chapter: 11th Chemistry : UNIT 6 : Gaseous State
Ideal gas equation
Ideal
gas equation
The gaseous state is described completely using the
following four variables T, P, V and n and their relationships were governed by
the gas laws studied so far.
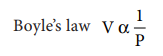
Charles law V α T
Avogadro’s law V α n
We can combine these equations into the following general
equation that describes the physical behaviour of all gases.
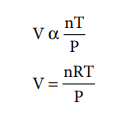
where, R is
the proportionality
constant called universal gas constant.
The above equation can be rearranged to give the ideal gas
equation
PV = nRT. ------ (6.11)
We already know that pressure is expressed in many
different units (Table 6.1) hence it is important to know the values of gas
constant R in different units as well.
We can calculate R using the equation,
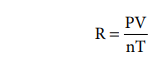
For Conditions in which P is 1 atm., volume 22.414 dm3. for 1 mole at 273.15 K.
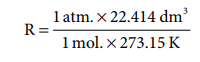
= 0.0821 dm3 atm. mol–1
K–1
Under standard conditions (STP) Where P = 1 bar (105
pascal), V= 22.71 Ă— 10-3 m3 for 1 mole of a gas at 273.15
K
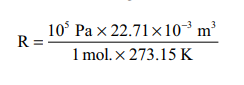
= 8.314 Pa m3 K–1
mol–1
= 8.314 × 10–5 bar m3 K–1 mol–1
= 8.314 × 10–2 bar dm3
K–1 mol–1
= 8.314 × 10–2 bar L K–1
mol–1
= 8.314 J K–1 mol–1
The ideal gas equation is a relationship between four
variables (P, V, T, n). Since it describes the state of any gas, it is referred
to as the equation of state of gases.
Let us calculate the pressure exerted by 2 moles of
sulphur hexafluoride in a steel vessel of volume 6 dm3 at 70 °C
assuming it is an ideal gas.
We will use the ideal gas equation for this calculation as
below:
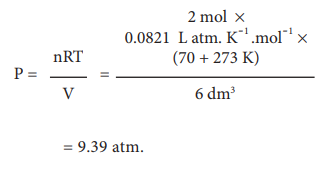
= 9.39 atm.
Related Topics