Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Kohlraush's Law - Application with example
KOHLRAUSH'S LAW
This law states that, ''at infinite dilution
wherein the ionisation of all electrolytes is complete, each ion migrates
independently and contributes a definite value to the total equivalent
conductance of the electrolyte''. Consider an electrolyte AB in aqueous
solution. It dissociates as
AmBn --- --- > mAn+ + nBm-
Then at infinite dilution, according to Kohlrausch's
law, the total equivalent conductance of the electrolyte, λ ∞ = (1/n+
) λA+ + (1/m- ) λB- where λ∞+ and λ∞- are the cationic and anionic equivalent
conductances at infinite dilutions and n+ and m- correspond the valency of
cations and anions furnished from each molecule of the electrolyte.
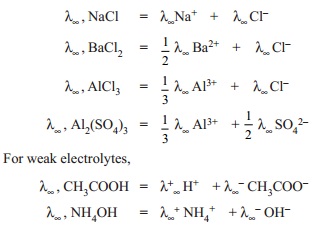
Application
of Kohlraush's law : The important use of
Kohlraush's law is to deduce the l¥
value of the weak electrolytes correctly by arithmetically combining the l¥
values of strong electrolyte in appropriate manner.
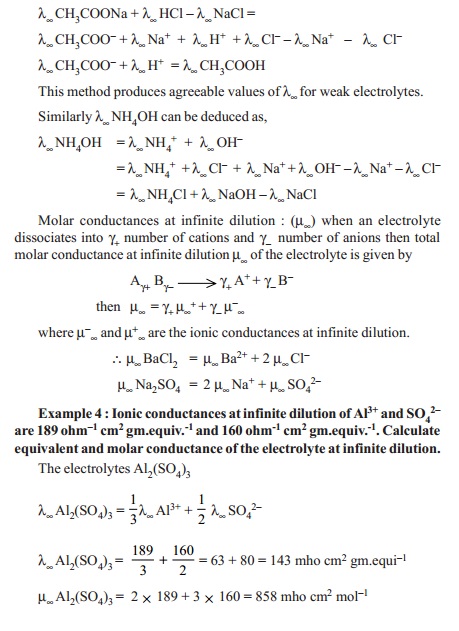
For example λ∞ of CH3COOH
which is a weak electrolyte is deduced from λ∞ values
of NaCl, HCl, and CH3COONa in such a manner that λ∞ of CH3COOH is obtained. Sodium
acetate (CH3COONa) is a strong electrolyte and it ionises to acetate
(CH3COO- ) and sodium (Na+) ions at all
concentrations in water. Applying Kohlraush's law,
Molar conductances at infinite dilution : (m¥)
when an electrolyte dissociates into g+ number of cations and g-
number of anions then total molar conductance at infinite dilution m¥
of the electrolyte is given by
A γ+ B γ - ----
> g+ A+ + γ - B-
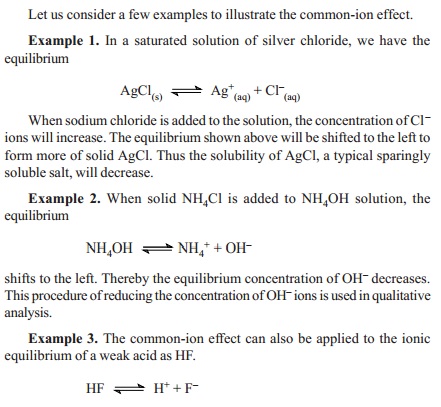
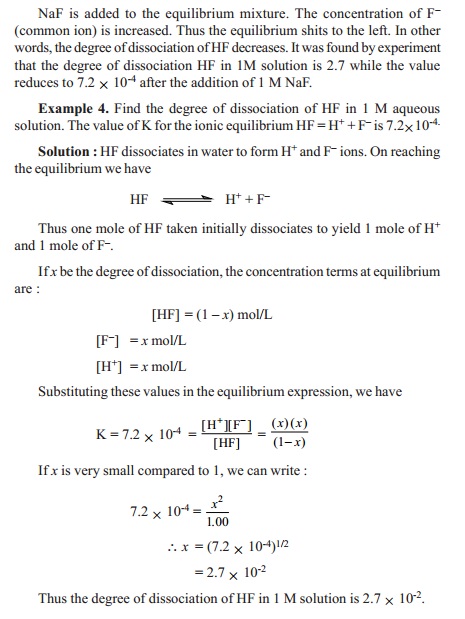
The common ion effect
When a soluble salt (say A+C-
) is added to a solution of another salt (A+B- )
containing a common ion (A+), the dissociation of AB is suppressed.
AB < ---
> A+ + B-
By the addition of the salt (AC), the
concentration of A+ increases. Therefore, according to Le Chatelier's
principle, the equilibrium will shift to the left, thereby decreasing the
concentration of A+ ions or the degree of dissociation of AB will be
reduced.
The
reduction of the degree of dissociation of a salt by the addition of a
common-ion is called the Common-ion effect.
Related Topics