Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Ionic Product Of Water : The pH of solutions
IONIC PRODUCT OF WATER
Water is a weak electrolyte. The dissociation
equilibrium of water can be considered as,
2H2O < --- -- > H3O+
+ OH-
According to law of mass action,
Keq = [H30+][OH-] / [H20]2
Since water as a solvent is always in excess
and change in concentration due its dissociation is negligible. Hence water
concentration is assumed to be constant.
Keq [H2O]2 = [H3O+]
[OH- ] = Kw
The constant Kw is called as the
ionic product of water and its value is given by the product of concentrations
of hydronium (H3O+) and hydroxide (OH- ) ions.
At 298 K, Kw = 1 ´ 10-14 mol2.dm-6.
The pH of solutions
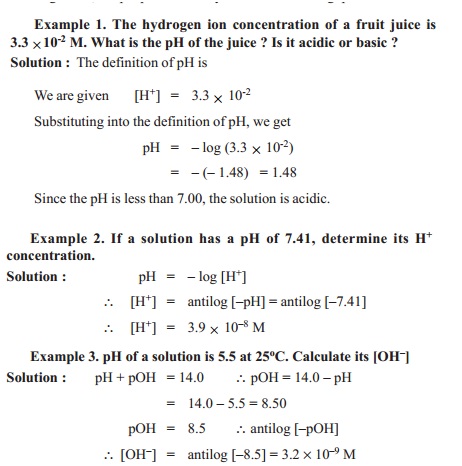
A knowledge of the concentration of hydrogen ions (more specifically hydronium ions) is of the greatest importance in chemistry. Hydrogen ion concentrations are typically quite small numbers. Therefore, chemists report the hydrogen ion concentration of a solution in terms of pH. It is defined as the negative of the base-10 logarithm (log) of the H+ concentration.
Mathematically it may be expressed as
pH = - log 10 [H+]
where [H+] is the concentration of
hydrogen ions in moles per litre Alternative and more useful forms of pH
definition are :
pH = - log 10 ( 1/[H+])
[H+] = 10-pH
The pH concept is very convenient for
expressing hydrogen ion concentration. It was introduced by Sorensen in 1909.
It is now used as a general way of expressing other quantities also, for
example.
(a) Concentration of OH- ions in aqueous solution of a base is
expressed as
p [OH-] = - log10 [OH-]
(b) Equilibrium constant for water is written
as
pKw = - log 10 [Kw]
For any quantity X, we can write
pX = - log X
The 'p' in these expressions means ''-log of
the quantity''.
The pH of a given solution can be measured with
the help of an apparatus called pH meter.
Knowing the pH of the solution its hydrogen ion
concentration can be calculated.
pH Scale
In order to express the hydrogen ion
concentration or acidity of a solution, a pH scale was evolved. The pH is
defined as
pH = - log [H +] or [H+] = 10-pH
The hydrogen ion concentrations of different
acidic solutions were determined experimentally. These were converted to pH
values using the above relations. Then these pH values were computed on a scale
taking water as the reference substance. The
scale on which pH values are
computed is called the pH
scale.
Water dissociates to H+ and OH-
ions to a very small degree so that we have the equilibrium.
H2O
< --- -- > H+ + OH-
Since Kw = 1 ´ 10-14
mol2.dm-6.
[H3O+] = [H+]
= [OH- ] = sq.rt(1x10-14) = 1x 10-7mol.dm-3
Thus the H+ ion and OH- ion
concentrations in pure water are both 10-7 mol.dm-3 at 25oC
and it is said to be neutral. In acidic solution, however, the concentration of
H+ ions must be greater than 10-7 mol. L-1.
Similarly in a basic solution, the concentration of OH- ions must be
greater than 10-7 mol L-1. Thus we can state :
neutral solution [H+] = [OH- ]
acidic solution [H+] > [OH- ]
basic solution [H+] < [OH- ]
Expressing the [H+] in terms of pH
for the different solutions cited above, we get what we call the pH scale. On
this scale the values range from 0 to 14. Since pH is defined as -log [H +]
and the hydrogen ion concentration of water is 10-7, the pH of water
is 7. All solutions having pH less than 7 are acidic and those with pH greater
than 7 are basic.
pH= 1 < -- --- ACID ---- > pH= 7
< -- BASE --- > pH= 14
As shown by the pH scale, pH decreases with the
increase of [H+]. The lower the pH, higher is the [H+] or
acidity.
To calculate [H+] and [OH-
] from Kw. In any aqueous solution, the product of [H+]
and [OH- ] always equal to Kw. This is so irrespective of the solute
and relative concentrations of H+ and OH- ions. However,
the value of Kw depends on temperature. At 25oC it is 1.0 ´ 10-14.
Thus,
[H+] [OH- ] = 1.0 ´ 10-14
Each of [H+] and [OH- ]
in pure water at 25oC is 10-7. The concentrations of [H+]
and OH- ions are expressed in gram moles per litre.
The concentrations [H+] and [OH-
] ions can be calculated from the expressions :
[H+]
= Kw / [OH-]
[OH- ] = Kw / [ H+ ]
Relation
between pH and pOH
pH concept can be used to express small
quantities as [OH- ] and Kw. Thus
pOH = - log10[OH-
]
pKw = - log
10 Kw
Let us consider the log form of the expression
K = [H+] [OH- ]
That is log K = log [H+] + log [OH-
]
- log K w = -log [H +] - log [OH -
]
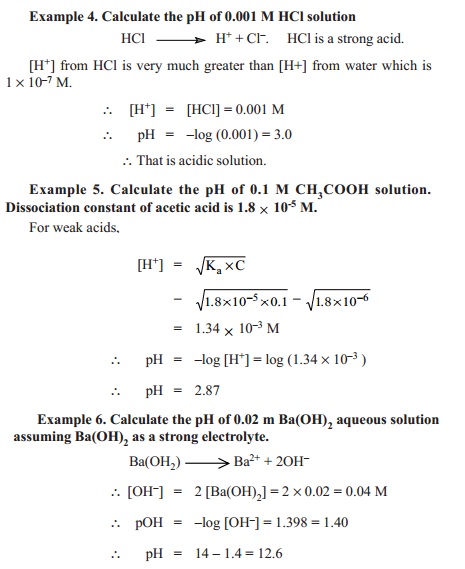
Thus pKw = pH + pOH
Since Kw = 1.0 ´ 10-14
pKw = -log (1.0x10-14 ) =
14.00
Hence, for any aqueous solution at 25oC,
pH and pOH add up to 14.00. That is,
pH + pOH = 14.00
Related Topics