Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Buffer Solutions
BUFFER SOLUTIONS
It is often necessary to maintain a certain pH
of a solution in laboratory and industrial processess. This is achieved with
the help of buffer solutions, buffer systems or simply buffers.
A buffer solution is one which maintains its pH
fairly constant even upon the addition of small amounts of acid or base.
In other words, a buffer solution resists (or
buffers) a change in its pH. That is, we can add a small amount of an acid or
base to a buffer solution and the pH will change very little. Two common types
of buffer solutions are :
i.
a weak acid together with a
salt of the same acid with a strong base. These are called Acid buffers.
(e.g.,) CH3COOH + CH3COONa.
ii.
a weak base and its salt with
a strong acid. These are called Basic buffers. (e.g.,) NH4OH + NH4Cl.
Buffer action : Let us illustrate buffer action by taking
example of a common buffer system
consisting of a solution of acetic acid and sodium acetate (CH3COOH/CH3COONa).
CH3COOH < --- --- > H+
+ CH3COO-
CH3COONa < -- --- > Na+ + CH3COO-
since the salt is completely ionised, it
provides the common ions CH3COO- in excess. The common
ion effect suppresses the ionisation of acetic acid. This reduces the
concentration of H+ ions which means that pH of the solution is
raised.
It is stated that a buffer solution containing
equimolar amounts (0.10 M) of acetic acid and sodium acetate has pH 4.74. Now
we proceed to discuss how the addition of a small amount of HCl or NaOH to the
buffer solution affects its pH.
The pH of the buffer is governed by the
equilibrium
CH3 COOH < -- --- > CH3
COO- + H+
The buffer solution has a large excess of CH3COO-
ions produced by complete ionisation of sodium acetate,
CH3 COONa -- --
-- > CH3 COO-+ Na+
1. Addition of HCl. Upon the addition of HCl, the decrease of H+ ions is counteracted by association with the excess of acetate ions to
form unionised CH3COOH. Thus the added H+ ions are
neutralised and the pH of the buffer solution remains unchanged. However owing
to the increased concentration of CH3COOH, the equilibrium (1)
shifts slightly to the right to increase H+ ions.
This explains the marginal increase of pH of
the buffer solution on addition of HCl.
2. Addition of NaOH. When NaOH is added to the buffer solution, the additional OH- ions combine
with CH3COOH to give CH3COO- and H2O.
Thus pH of the buffer solution is maintained almost constant. The buffer NH4OH/NH4Cl
can also be explained on the same lines as of an acid buffer upon addition of
HCl the H+ ions combine with NH4OH to form NH4+
and H2O. pH is retained. Similarly when NaOH is added, the OH-
ions combine with NH4+ ions present in the buffer
solution to give NH4OH and hence pH is maintained.
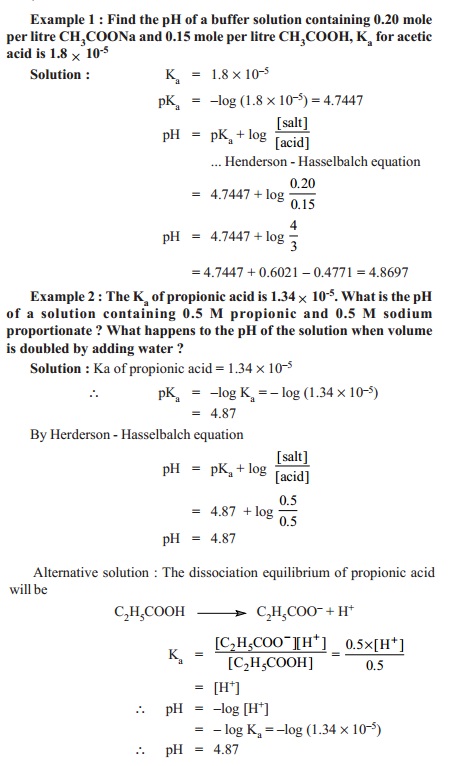
Henderson
equation : The pH of an acid buffer can
be calculated from the dissociation
constant, Ka, of the weak acid and the concentrations of the acid
and the salt used.
The dissociation expression of the weak acid,
HA, may be represented As
HA < -- -- -- > H+ + A-
Ka = [H+][A-]
/ [HA]
[H+] = [HA] Ka / [A-] ……………… 1
The weak acid is only slightly dissociated and
its dissociation is further depressed by the addition of the salt (Na+
A- ) which provides A- ions (Common ion effect). As a
result the equilibrium concentration of the unionised acid is nearly equal to
the initial concentration of the acid. The equilibrium concentration [A-
] is presumed to be equal to the initial concentration of the salt added since
it is completely dissociated. Thus we can write the equation (1) as
[H+] = Ka ([acid] / [salt]
) ….. ..2
where [acid] is the initial concentration of
the added acid and [salt] that of the salt used.
Taking negative logs of both sides of the
equation (2), we have
-log[H+] = -logKa - log ([acid]/[salt])
-log[H+] = pH and -logKa =
pKa
pH = pKa + log ([salt]/[acid])
This relationship is called the Henderson-Hasselbalch equation or simply Henderson equation.
In a similar way, the Henderson-Hasselbalch
equation for a basic buffer can be derived. This can be stated as :
pOH = pKb +
log ([salt]/[acid])
![]() Significance of the Henderson-Hasselbalch
equation. With its help
Significance of the Henderson-Hasselbalch
equation. With its help
1. The pH of a buffer solution can be
calculated from the initial concentrations of the weak acid and the salt
provided Ka is given.
However, the Henderson-Hasselbalch equation for
a basic buffer will give pOH and its pH can be calculated as (14 - pOH).
2. The dissociation constant of a weak acid (or
weak base) can be determined by measuring the pH of a buffer solution
containing equimolar concentrations of the acid (or base) and the salt.
Since, [salt] = [acid], log ([salt]/[acid]) = log 1 =0
pKa = pH
The measured pH, therefore, gives the value of
pKa of the weak acid.
Likewise we can find the pKb of a
weak base by determining the pOH of equimolar basic buffer.
3. A buffer solution of desired pH can be
prepared by adjusting the concentrations of the salt and the acid added for the
buffer.
It is noteworthy that buffer solutions are most
effective when the concentrations of the weak acid (or weak base) and the salt
are about equal. This means that pH is close to the value of pKa of
the acid (or pKb of the base).
Related Topics