Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Henderson-Hasselbalch equation and its Significance
Henderson
equation : The pH of an acid buffer can
be calculated from the dissociation
constant, Ka, of the weak acid and the concentrations of the acid
and the salt used.
The dissociation expression of the weak acid,
HA, may be represented As
HA < -- -- -- > H+ + A-
Ka = [H+][A-]
/ [HA]
[H+] = [HA] Ka / [A-] ……………… 1
The weak acid is only slightly dissociated and
its dissociation is further depressed by the addition of the salt (Na+
A- ) which provides A- ions (Common ion effect). As a
result the equilibrium concentration of the unionised acid is nearly equal to
the initial concentration of the acid. The equilibrium concentration [A-
] is presumed to be equal to the initial concentration of the salt added since
it is completely dissociated. Thus we can write the equation (1) as
[H+] = Ka ([acid] / [salt]
) ….. ..2
where [acid] is the initial concentration of
the added acid and [salt] that of the salt used.
Taking negative logs of both sides of the
equation (2), we have
-log[H+] = -logKa - log ([acid]/[salt])
-log[H+] = pH and -logKa =
pKa
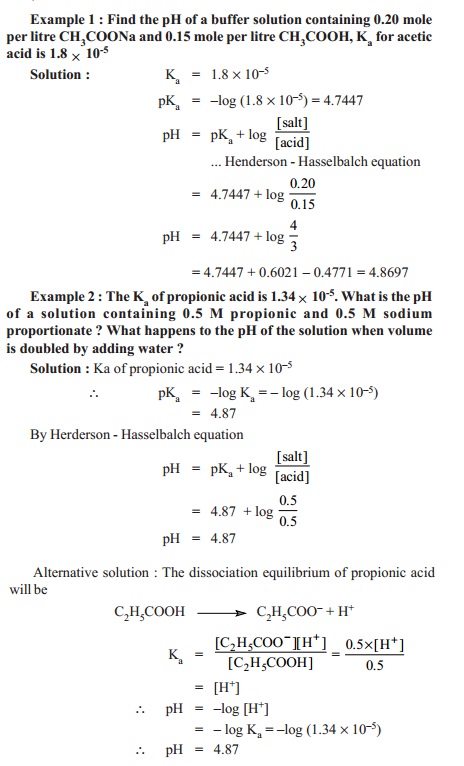
pH = pKa + log ([salt]/[acid])
This relationship is called the Henderson-Hasselbalch equation or simply Henderson equation.
In a similar way, the Henderson-Hasselbalch
equation for a basic buffer can be derived. This can be stated as :
pOH = pKb +
log ([salt]/[acid])
![]() Significance of the Henderson-Hasselbalch
equation. With its help
Significance of the Henderson-Hasselbalch
equation. With its help
1. The pH of a buffer solution can be
calculated from the initial concentrations of the weak acid and the salt
provided Ka is given.
However, the Henderson-Hasselbalch equation for
a basic buffer will give pOH and its pH can be calculated as (14 - pOH).
2. The dissociation constant of a weak acid (or
weak base) can be determined by measuring the pH of a buffer solution
containing equimolar concentrations of the acid (or base) and the salt.
Since, [salt] = [acid], log ([salt]/[acid]) = log 1 =0
pKa = pH
The measured pH, therefore, gives the value of
pKa of the weak acid.
Likewise we can find the pKb of a
weak base by determining the pOH of equimolar basic buffer.
3. A buffer solution of desired pH can be
prepared by adjusting the concentrations of the salt and the acid added for the
buffer.
It is noteworthy that buffer solutions are most
effective when the concentrations of the weak acid (or weak base) and the salt
are about equal. This means that pH is close to the value of pKa of
the acid (or pKb of the base).
Related Topics