Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Hybridization : Salient Features, Type, Example
HYBRIDISATION
Hybridization is the concept of intermixing of the orbitals of an atom having nearlythesameenergytogiveexactlyequivalentorbitalswithsameenergy,identical shapes and symmetrical orientations in space.
The new equivalent orbitals formed are known as the hybrid orbitals or hybridized orbitals. Hybrid orbitals have properties entirely different from the
properties of the original orbitals from which they have
been obtained.
Salient Features regarding Hybridisation
1.
Orbitals involved
in hybridization should have nearly the same energy.
2.
The orbitals of
one and the same atom participate in hybridization.
3.
The number of
hybrid orbitals formed is equal to the number of hybridizing orbitals.
4.
The hybrid orbitals
are all equivalent in shape and energy.
5.
A hybrid orbital
which is taking part in bond formation must contain one electron in it.
6.
Due to the
electronic repulsions between the hybrid orbitals, they tend to remain at the maximum distance apart.
7.
The head on
overlap of atomic orbitals give sigma (s) bonds.
8.
The sidewise or
lateral overlap of atomic orbitals give pi (p) bonds.
Tips to Predict the Type of Hybridisation in a Molecule or Ion (Other than Complex Ions)
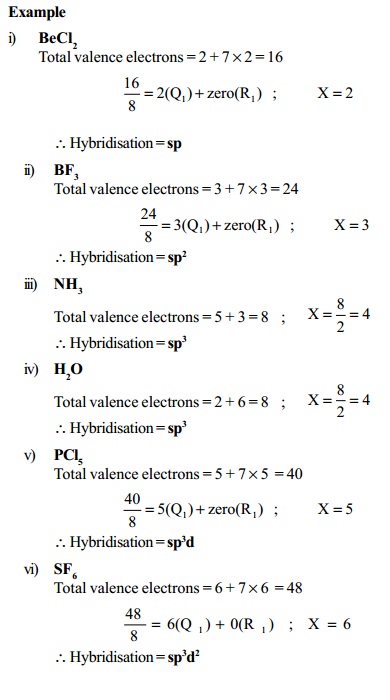
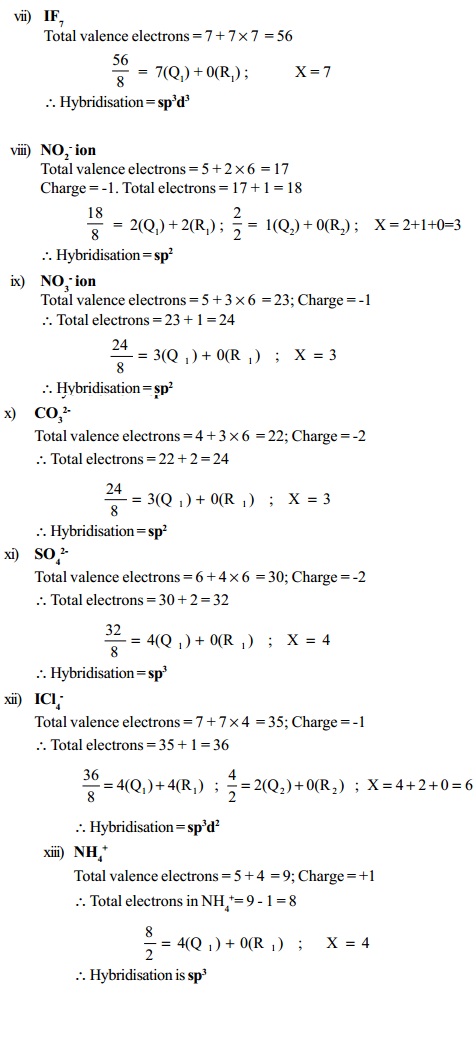
Step 1 : Add the number of valence electrons of all the atoms present in the given molecule/ion.
Step 2 : In case of a cation, subtract the number of electrons equal to the charge on the cation and in case of an anion, add number of electrons equal to the charge on the anion.
Step 3 : (i) If the result obtained in step 2 is less than 8, divide it by 2 and find the sum of the quotient and remainder.
(ii) If the result obtained in step 2 lies between 9 and 56, divide it by 8 and find the first quotient (Q1). Divide the remainder R1 (if any) by 2 and find the
second quotient (Q2). Add all the quotients and the final
remainder (R2).
Let the final result obtained in (i) or (ii) be X. The type of hybridisation is decided by the value of X as follows :
Value of X : Type of hybridization
2 : Sp
3 : sp2
4 : sp3
5 : sp3d
6 : sp 3 d 2
7 : sp3d3
Example
i) BeCl2
Total valence electrons = 2 + 7 ×
2 = 16
16
/ 8 = 2(Q1 ) + zero(R1) ; X=2
Hybridisation
= sp
ii) BF3
Total valence electrons = 3 + 7 ×
3 = 24
24 / 8 = 3(Q1 ) +
zero(R1) ; X=3
Hybridisation in some Typical Molecules and Ions
Hybridisation
sp - Be F2, BeCl2, C2H2,
CO2
sp2 - SO2, BH3, BF3, NO2-, NO3-, CO32-
sp3 - NH3, H2O, CH4, CCl4, SiCl4, H3O+,NH4+, ClO2-, ClO3-, ClO4-,NF3
sp3d - PCl5, ClF3, SF4, XeF2
sp3d2
- SF6, XeF4, XeOF4, BrF5
sp3d3
- IF7, XeF6
Related Topics