Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Importance, Strength, Types of Hydrogen bonds - Intermolecular Forces
INTERMOLECULAR
FORCES
The ionic, covalent and
coordinate bond arises due to attractive forces between atoms. Vander Waal (Dutch physicist, 1873) was the first to
propose the existence of attractive forces between the atoms of
inert gases with fully filled orbitals.
These forces also exist between non-polar molecules as well as polar molecules. The attractive interactions between the
molecules are responsible for bringing the
molecules close together. The attractive interactions between the different molecule of a substance are called
intermolecular forces. The magnitude of
these forces is maximum in the solids and decreases on passing from solid to liquids and from liquid to gaseous state. Vander Waal
successfully explained the liquefaction
of gases on the basis of inter molecular forces. These forces are purely electrostatic and thus physical in nature.
Hydrogen bonding.
Hydrogen bonding comes into existence as a result of dipole-dipole interactions between the molecule in which
hydrogen atom is covalently bonded
to a highly electronegative atom. Therefore, the conditions for the effective
hydrogen bonding are :
i) high electronegativity of the atom bonded to hydrogen
atom so that bond is sufficiently
polar.
ii) small size of the atom bonded to hydrogen so that it
is able to attract the bonding electron
pair effectively.
If the atom bonded to hydrogen has low value of
electronegativity and/or large atomic size,
dipole-dipole interactions are not strong enough to allow effective hydrogen bonding.
Only nitrogen, oxygen and fluorine form strong hydrogen
bonds because they have high value of
electronegativity and small atomic size.
Strength of H-bonds. It is a weak bond because it is merely an electrostatic force and not
a chemical bond. Its strength depends upon the electronegativity of atom to which H atom is ovalently bonded. Since
electronegativity of F > O >N,
the strength of H- bond is in the order H - F ......... H > H-O.....H >
H- N.....H. Hydrogen bonds are much weaker than covalent
bonds. The bond strength of
different bonds is in the order : Ionic bond > Covalent bond >Hydrogen bond > dipole-dipole interactions, Vander
Waal's (London forces).
Types of Hydrogen bonds
There are two different types of hydrogen bonds as :
i)
Intermolecular hydrogen bonding. This type of bond is
formed between the two molecules of the
same or different compounds. Some examples of the compounds exhibiting intermolecular hydrogen bonds are :
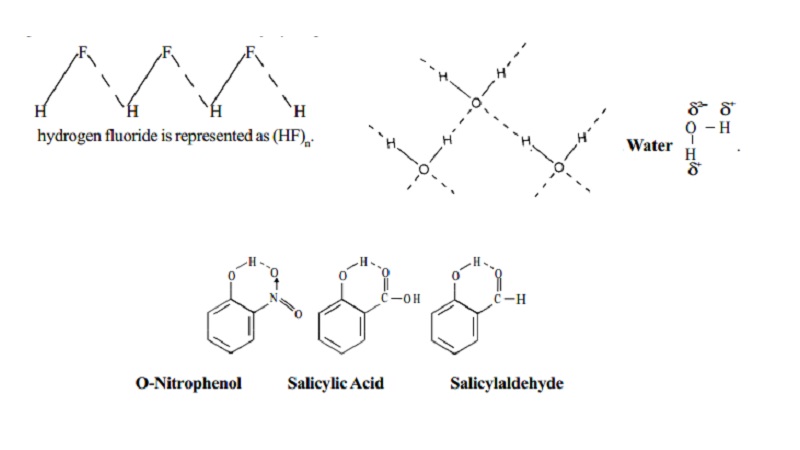
d+ d-
1. Hydrogen
fluoride, H - F. In the solid
state, hydrogen fluoride consists of
long zig-zag chains of molecules associated by hydrogen bonds as shown below :
Therefore, hydrogen fluoride is represented as (HF)n.
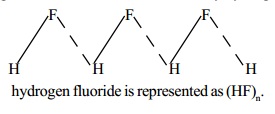
2. Water In
water molecule, the electronegative oxygen atom forms two polar covalent bonds
with two hydrogen atoms. The oxygen atom due
to its higher electronegativity acquires partial negative charge and the two hydrogen atoms acquire partial positive charge. The
negatively charged oxygen forms two hydrogen bonds with two positively charged
hydrogen atoms of two neighbouring molecules. Each oxygen atom is tetrahedrally
surrounded by four hydrogen atoms as
shown below :Hydrogen bonding in water results in a hydrogen bridge (H-O-H)
network extending in three dimensions and the associated water
molecule may be expressed
as (H2O)n.
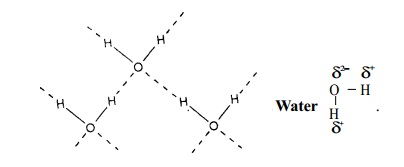
ii) Intramolecular hydrogen bonding. This type of bond is formed between hydrogen atom and N, O or F atom of the same
molecule. This type of hydrogen bonding
is commonly called chelation and is more frequently found in organic compounds. Intramolecular hydrogen bonding is
possible when a six or five membered rings can be formed.
Intramolecular hydrogen bonding (chelation) decreases the
boiling point of the compound and
also its solubility in water by restricting the possibility of inter- molecular hydrogen bonding.
Importance of H-bonding
i) Life would have been impossible without liquid water
which is the result of intermolecular H-bonding in it.
ii) Hydrogen bonding increase the rigidity and strength
of wood fibres and thus makes it an
article of great utility to meet requirements of housing, furniture, etc.
iii) The cotton, silk or synthetic fibres also own their
rigidity and tensile strength to hydrogen
bonding.
iv) Most of our food materials such as carbohydrates and
proteins also consist
of hydrogen bonding.
v) Hydrogen bonding also exists in various tissues,
organs, skin, blood and bones.
Related Topics