Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Group 13 - Boron Group (B, Al, Ga, In, Tl): Extraction, Properties
Group 13 - Boron Group (B, Al, Ga, In, Tl)
Boron does not occur in the free state in
nature. In the combined state, it occurs mainly in the form of the salts of
boric acid.
Ores of Boron
i) Boric
acid H3BO3
ii) Borax Na2B4O7.10H2O
Extraction
On a large scale, boron is extracted from its minerals, borax Na2B4O7
or colemanite Ca2B6O11. The latter is first
converted to borax by boiling with a solution of sodium carbonate in the
requisite proportion.
2Ca2B6O11 + 3Na2CO3
+ H2O -- > 3Na2B4O7 +
3CaCO3 + Ca(OH)2 The insoluble calcium carbonate settles
down and borax is crystallized
from the
mother liquor. Boron is isolated from borax in the following two steps.
a) Preparation of
boron trioxide:- Borax is treated with hot concentrated hydrochloric acid, when the sparingly soluble boric
acid slowly separates out.
Na2B4O7 +
2HCl -- > 2NaCl + H2B4O7 H2B4O7 + 5H2O -- > 4H3BO3
Boric acid is strongly heated when borontrioxide is obtained 2H3BO3 -- > B2O3 + 3H2O
b) Reduction of
borontrioxide:- A mixture of borontrioxide with sodium, potassium or magnesium pieces
is heated in a crucible to bright redness. The residual boron is broken up and
boiled with concentrated HCl to dissolve out magnesium oxide and excess of
boric acid when a dark brown powder of amorphous boron is obtained as a
residue: It is washed with water and dried.
B2O3 + 3Mg
-- > 2B + 3MgO.
Pure boron is obtained in the crystalline form by passing a mixture of
boron tribromide vapours and hydrogen over electrically heated filament of
tungsten at 1470K. It may also be prepared by submitting a mixture of
borontrichloride vapour and hydrogen to the action of a high tension electric
arc, when boron is obtained on cooling as a hard black amorphous mass.
Physical properties
Boron
exists in two allotropic forms amorphous and crystalline boron. Boron is a
non-metallic element and is a non-conductor of electricity.
Chemical properties
1. Action of air:- It is unaffected by air at ordinary temperature but when heated in air to about 975K, it burns
forming boron trioxide and a little boron nitride, BN
4B + 3O2 -- > 2B2O3
2B + N2 -- > 2BN
2. With acids: - Amorphous boron dissolves in hot concentrated sulphuric and in nitric acid to form boric acid.
B + 3HNO3 -- > H3BO3 + 3NO2
2B + 3H2SO4
-- > 2H3BO3 + 3SO2.
3. With caustic alkali:- It dissolves in fused caustic alkali and forms boric acid.
4.
As a reducing agent:- Boron is a
powerful reducing agent and can even
replace carbon from carbon dioxide and silicon from silica.
3CO2 + 4B -- > 2B2O3 + 3C 3SiO2 + 4B -- > 2B2O3 + 3Si
5.
With metals:- It combines with metals (except Cu, Ag and Au) at
high temperature in the electric
furnace to form borides.
6. With non-metals:- Boron combines with nitrogen, chlorine, bromine and carbon at higher temperature forming boron nitride, BN, boron
trichloride, BCl3, boron tribromide, BBr3 and boron carbide, B4C respectively.
Boron carbide is probably the hardest substance known.
Compounds of Boron
Borax (or) Sodium
tetraborate, Na2B4O7 - Tincal, a crude form of borax,
contains 55% of it and is found in the land dried up lakes of Tibet.
Borax can
be prepared
From colemanite:- It is boiled with
concentrated solution of sodium carbonate.
Ca2B6O11
+ 2Na2CO3 -- > 2CaCO3 + Na2B4O7
+ 2NaBO2.
On filtration and concentration, crystals of borax separate. A current
of CO2 is passed through the mother liquor to convert the metaborate into
borax.
4NaBO2
+ CO2 -- > Na2CO3 + Na2B4O7
The residual sodium carbonate is used again for the treatment of a fresh
quantity of colemanite.
From Tincal - Naturally
occurring crude borax (Tincal) is dissolved in water, filtered, concentrated and crystallized when pure borax is
obtained.
Properties
When
borax is heated above its melting point until all the water of crystallization
is expelled, it forms a colourless glassy substance known as borax glass. It
then decomposes to give sodium meta borate and boron (III) oxide.
Na2B4O7.10H2O
-- equ-- > ®Na2B4O7 +
10H2O
Na2B4O7--
- eq-- > 2NaBO2
+ B2O3
When this mixture is fused with metallic oxide
it forms characteristic coloured beads. With the help of the colour, the metal
ions can be identified. For example
CuO + B2O3
-- > Cu(BO2)2.
Uses: Borax is
used
1. to identify the metallic radicals in the qualitative analysis
2. as a flux in welding metals
3. in the manufacture of glass, soap and porcelin
4. as cleaning and dyeing agent in tanneries
5. as a food preservative.
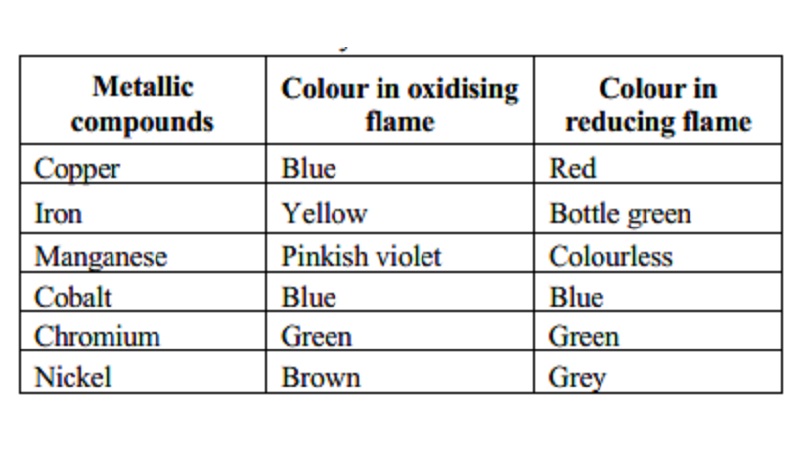
Borax bead test
A pinch of
borax is heated in a platinum loop, it melts to give a colourless glassy bead.
It is then dipped in a coloured metallic salt solution and again heated.
Characteristic coloured beads are formed. From the colour of the beads, the
basic radicals are identified. Due to the formation of metallic metaborate, the
characteristic colours are formed.
Example: Copper salts give blue beads
In an oxidising flame
CuSO4
+ B2O3 -- > Cu(BO2)2 + SO3
In a reducing flame
2Cu(BO2)2
+ C -- > 2CuBO2 + B2O3
+ CO 2CuBO2 + C -- > 2Cu + B2O3 + CO
Borax bead
test is used to identify the coloured salts.
Related Topics