Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
p-Block Elements - General Characteristics
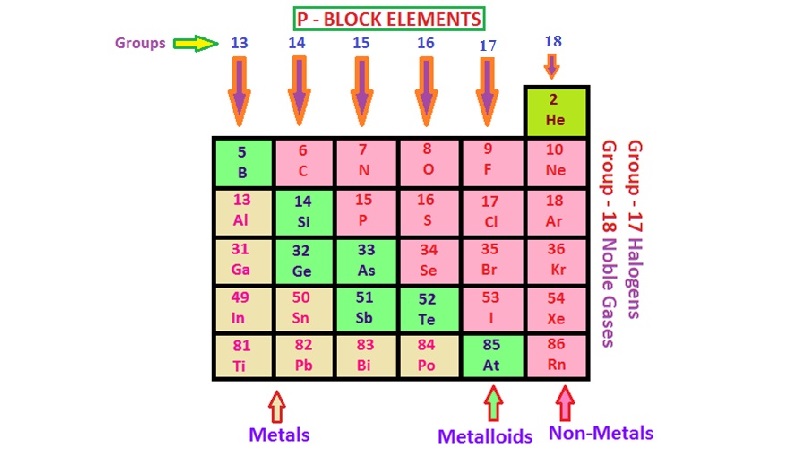
p-BLOCK ELEMENTS
General Characteristics
The elements belonging to the group 13 to 18 of the periodic table, in which
p-orbitals are progressively filled are collectively known as p-block elements.
In all these elements while s-orbitals are completely filled, their
p-orbitals are incomplete. These are progressively filled by the addition of
one electron as we move from group 13 (ns2np1) to group
17 (ns2np5). In group 18 (ns2np6)
both s and p-orbitals are completely filled.
p-block
elements show a variety of oxidation state both positive and negative. As we go
down the group, two electrons present in the valence `s' orbital become inert
and the electrons in the `p' orbital are involved in chemical combination. This
is known as `inert pair effect'.
The inert pair effect is really a name, not an
explanation. A full explanation involves the decreasing strength of the M-X
bond going down the group (for covalent compounds) or the decreasing lattice
energies of compounds containing the M4+ ion (for ionic compounds).
In this way the energy input needed to form compounds of the formula MX4
are less likely to be balanced by the energy released when the four M-X bonds
are formed, so the equilibrium favours the left hand side.
MX2 + X2 -- > MX4
The existence of a positive oxidation state corresponding to the group
number and of another state two units lower is an illustration of the inert
pair effect, the term referring to the valence `s' electrons, used in bonding
in the higher oxidation state but not in the lower.
With the increase in atomic mass, the ionic
character of bonds of the compounds of the group 13 (IIIA) elements increases,
and some of the heavier metal ions do exist in the +3 oxidation state in
aqueous solution. The stability of such compounds with the +3 oxidation state
is, however, lower than those with the +1 oxidation state in the case of
heavier members of this group. Thus thallium in +1 oxidation state is more
stable than in +3 state. This is because, the s electrons in the ns sub-shell
do not prefer to form bonds.
This inertness is found only, i) when the `s' electrons are in the fifth
or higher principal quantum number ii) when their loss does not afford a
species with a noble gas configuration. This property of stabilising the lower
oxidation state keeping the paired electron in the ns orbital is referred to as
the `inert pair effect'. This effect is also observed in the elements of groups
12 (IIB), 14(IVA) and 15(VA) where the heavier elements exhibit 0, +2 and +3 oxidation
states respectively.
Nature of oxides
Oxides of p-block elements may be basic (in case
of metallic elements), amphoteric (in case of metalloids) or acidic (in case of
non-metals). Non-metals also form a number of oxyacids. In all the groups, the
acidic character of the oxide decreases as we move down the group while it
increases in the same period from left to right.
For
example
Basic
oxide - Bi2O3
Amphoteric
oxide - SnO, SnO2, PbO,
Pb2O3
Acidic
oxides - SO3, Cl2O7
Oxyacids - HNO3,
H2SO4.
Basic character increases down the
group
CO2 SiO2 GeO2 SnO PbO
Acidic less acidic amphoteric basic most basic
Acidic character increases across a
period
Al2O3 SiO2 P4O10
SO2 Cl2O7
amphoteric
acidic most acidic
Nature of hydrides
Many of the p-block elements form hydrides. The hydrides of non-metals
are more stable. Thus in any group the stability of the hydride decreases from
top to bottom; its strength as an acid also increases in this order. Thus among
all the hydrides, hydrogen iodide forms the strongest acid solution in water.
In group 15, nitrogen forms the stablest hydride of all. Thus the order of
stability of these hydrides is
NH3 > PH3 > AsH3 > SbH3 > BiH3
Nature of halides
Out of the
p-block elements, the non-metals form covalent halides. Metallic halides show a
gradation from an ionic character to covalent character. As we move from left
to right across the period, ionic character of the halides decreases and
covalent character increases. For example, SbCl2 is partially ionic whereas
TeCl4 is covalent.
In case metals forms halides in more than one oxidation states, halides
in lower oxidation state are largely ionic and those in higher oxidation state
are largely covalent.
Polarizability of a halide ion depends on its size. Iodides and bromides
are more covalent while fluorides are more ionic.
Related Topics