Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Carbon group elements: properties, Structure, Uses
Carbon group elements
The
elements carbon, silicon, germanium, tin and lead constitute the 14th
group of the periodic table. These are p-block elements having the
configuration ns2np2.
Element At.No. Electronic structure
Carbon 6 [He] 2s2
2p2
Silicon 14 [Ne] 3s2
3p2
Germanium 32 [Ar] 3d10 4s2 4p2
Tin 50 [Kr] 4d10
5s2 5p2
Lead 82 [Xe] 4f14
5d10 6s2 6p2
Allotropic forms of carbon
Carbon exhibits allotropy and occurs as
1.
Diamond, a beautiful crystalline substance
2.
Graphite, a soft greyish black crystalline
substance
Amorphous
carbon, black residue left when carbon compounds are heated.
Different amorphous
varieties of carbon are
(i) Coal,
(ii) Coke,
(iii) Charcoal,
(iv) Bone
black or animal charcoal,
(v) lampblack,
(vi)
carbon black,
(viii) Gas
carbon and
(ix)
petroleum coke.
Structure of diamond
In diamond
every atom is bonded with the other by covalent links resulting in the
formation of giant molecule. Each carbon atom is linked with four neighbouring
carbon atoms held at the corners of a regular tetrahedron by covalent bonds.
The C-C bonds are very strong. The crystal of diamond is very hard and has high
melting and boiling points.
The
combined strength of the many carbon-carbon bonds within the structure of
diamond give it both great hardness and a lack of chemical reactivity.
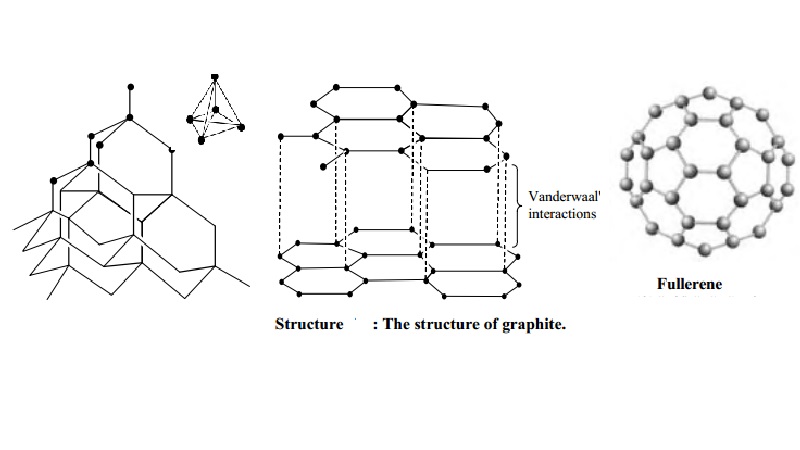
Structure of graphite
It
consists of separate layers. The carbon atoms are arranged in regular hexagons
in flat parallel layers. There is no strong bonding between different layers,
which are, therefore, easily separable from each other. Since there are no
covalent linkages between the adjacent planes, graphite can be easily cleaves
along the lines of the planes. Whilst the bonds within the layers are strong,
those between the layers are not and so they slide over each other easily This
accounts for the softness and lubricating power of graphite.
Structure of Buckminster fullerenes
Fullerenes
In 1985, a
new allotrope of carbon was discovered by Richard Smalley and Robert Curl of
Rice University, Texas, working with Harry Kroto of Sussex University. The
first to be identified and the most symmetrical of the family, with 60 atoms
and 32 sides (20 hexagons and 12 pentagons), was nick named `buckyball' and was
then named buck minister fullerene, because it resembles the geodesic domes
developed by an American inventor called R.Buckminister fuller. The group of
spherical carbon molecules is called fullerenes. These compounds have
superconducting properties and its potential for opening new areas of chemistry
have made study of the `buckyball' as one of the most rapidly expanding areas
of chemical research.
Amorphous form of carbon
Amorphous carbon is the most reactive form of
carbon. It burns relatively easily in air, thereby serving as a fuel, and is
attacked by strong oxidising agents. This form has structural features of
graphite, such as sheets and layers. It's atomic structure is much more
irregular.
General properties
Metallic character
Carbon and silicon are non-metals, germanium is
a metalloid while tin and lead are metals. Thus metallic character increases on
descending the group since ionization energy decreases on descending the group.
Hydrides
All of these elements form covalent hydrides
though the number of hydrides and the ease with which these are formed
decreases from carbon to lead. Carbon gives a vast number of hydrides (alkanes),
silicon and germanium (silanes and germanes) whereas stannane (SnH4) and
plumbane (PbH4) are the only hydrides of tin and lead are known.
Unlike
alkanes, silanes are strong reducing agents, explode in chlorine and are
readily hydrolysed by alkaline solutions. The difference is probably due to the
difference in electronegativity between C and Si resulting in difference
between C-H and Si-H linkages.
Halides
All these elements give tetrahalides.
Tetrachlorides are usually fuming liquids at ordinary temperature. Carbon
tetrahalide resists hydrolysis. This is because due to the absence of
d-orbitals. Maximum covalency of carbon is only four and there is no
possibility of formation of coordinate linkages with H2O, which could lead to
hydrolysis.
Tetrahalides of rest of the elements undergo hydrolysis. For example
SiX4 + 2H2O SiO2 + 4HX
Carbon, silicon and germanium form trihalides of the type MHX3. Lead and
tin do not form trihalides. Silicon, germanium, tin and lead form dihalides.
Chlorides
1.
The chlorides are all simple molecular
substances with tetrahedral molecules.
2.
The stability of the chlorides decreases down
the group and the +2 oxidation state becomes more stable than the +4 state.
Only tin and lead form chlorides in which their oxidation state is +2, the
other chlorides existing solely in the +4 state. Tin(II) chloride is a solid
that is soluble in water, giving a solution which conducts electricity. It is
also soluble in organic solvents. Its melting point is 246deg C. Lead(II) chloride is also a solid. It is
sparingly soluble in water. The chlorides of the group 14 elements in their +4
oxidation state illustrate further the change in character of the elements from
non-metal to metal down the group and giving a solution which conducts
electricity, and melts at 501deg C. These observations suggest that tin(II) chloride has both covalent
and ionic character, while lead(II) chloride is predominantly ionic.
3.
All the chlorides with +4 oxidation state are
readily hydrolysed by water, except tetrachloromethane (CCl4).
Carbides
Compounds
of carbon with less electronegative elements (eg. metals, Be, B, Si etc.) are
called carbides. These are of three main types.
i.
Ionic or salt-like eg. acetylides, methanides,
allylides
ii.
Interstitial or metallic eg. WC and
iii.
Covalent eg. B4C, SiC.
All the
three types of carbides are prepared by heating the element or its oxide with
carbon or a hydrocarbon to a high temperature.
2Be + C -- > Be2C
CaO + 3C -- > CaC2 + CO
SiO2 + 3C -- > SiC + 2CO
Oxides
1.
The oxides show a marked trend in structure from
the molecules of carbondioxide to giant structures intermediate between ionic
and covalent lower down the group.
2.
The +2 oxidation state is the more stable state
in the case of leadoxide, and lead (IV) oxide decomposes on heating giving
lead(II) oxide, a solid that melts at 886degC. The structure of lead(II) oxide is predominantly ionic.
3.
The oxides at the top of the group (CO2 and SiO2)
have an acidic nature, the carbonate ion CO32- being produced easily
in dilute aqueous solutions. The ease of formation of oxoanions (SiO32-,
GeO32-etc.) decreases down the group as the acidic character
decreases. The oxides of germanium, tin and lead are amphoteric, reacting to
form simple salts with acids.
Uses of carbon and its
compounds
1.
Carbon and its compounds play an enormous role
in the global economy, eg. Fossil fuels.
2.
Halogenated carbon compounds are used as
refrigerants, aerosol propellants, fire extinguisher and solvents.
3.
CS2 is used in the manufacture of
viscose rayon (artificial silk) and cellophane.
Related Topics