Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Structure of Diamond, Graphite, Buckminster fullerenes
Structure of diamond
In diamond
every atom is bonded with the other by covalent links resulting in the
formation of giant molecule. Each carbon atom is linked with four neighbouring
carbon atoms held at the corners of a regular tetrahedron by covalent bonds.
The C-C bonds are very strong. The crystal of diamond is very hard and has high
melting and boiling points.
The
combined strength of the many carbon-carbon bonds within the structure of
diamond give it both great hardness and a lack of chemical reactivity.
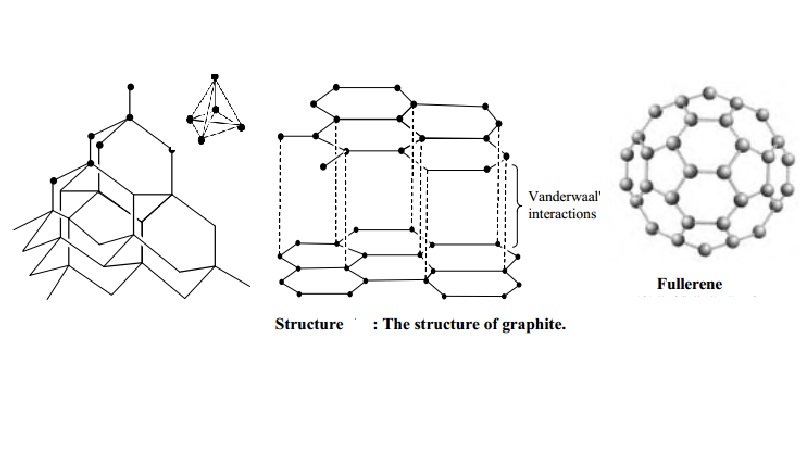
Structure of graphite
It
consists of separate layers. The carbon atoms are arranged in regular hexagons
in flat parallel layers. There is no strong bonding between different layers,
which are, therefore, easily separable from each other. Since there are no
covalent linkages between the adjacent planes, graphite can be easily cleaves
along the lines of the planes. Whilst the bonds within the layers are strong,
those between the layers are not and so they slide over each other easily This
accounts for the softness and lubricating power of graphite.
Structure of Buckminster fullerenes
Fullerenes
In 1985, a
new allotrope of carbon was discovered by Richard Smalley and Robert Curl of
Rice University, Texas, working with Harry Kroto of Sussex University. The
first to be identified and the most symmetrical of the family, with 60 atoms
and 32 sides (20 hexagons and 12 pentagons), was nick named `buckyball' and was
then named buck minister fullerene, because it resembles the geodesic domes
developed by an American inventor called R.Buckminister fuller. The group of
spherical carbon molecules is called fullerenes. These compounds have
superconducting properties and its potential for opening new areas of chemistry
have made study of the `buckyball' as one of the most rapidly expanding areas
of chemical research.
Related Topics