The Gas Laws - Gay-Lussac’s Law (Pressure-temperature relationship) | 11th Chemistry : UNIT 6 : Gaseous State
Chapter: 11th Chemistry : UNIT 6 : Gaseous State
Gay-Lussac’s Law (Pressure-temperature relationship)
Joseph Gay-Lussac stated that, at constant volume the pressure of a fixed mass of a gas is directly proportional to temperature.
Gay-Lussac’s Law (Pressure-temperature relationship)
Joseph Gay-Lussac stated that, at constant volume the pressure of a fixed mass of a gas is directly proportional to temperature.
P α T
or P/T = Constant k
If P1 and P2 are the pressures at temperatures T1 and T2, respectively, then from Gay Lussac’s law
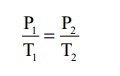
Tags : The Gas Laws , 11th Chemistry : UNIT 6 : Gaseous State
Study Material, Lecturing Notes, Assignment, Reference, Wiki description explanation, brief detail
11th Chemistry : UNIT 6 : Gaseous State : Gay-Lussac’s Law (Pressure-temperature relationship) | The Gas Laws
Related Topics
11th Chemistry : UNIT 6 : Gaseous State