Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Electrochemistry - Cells and Daniel cell
ELECTROCHEMISTRY - CELLS
In electrochemistry, the interconversion of chemical energy and electrical energy is an important aspect that possesses numerous applications. For example, batteries supply electrical energy stored in the form of chemical energy for the operation of torch, radio, calculators etc. Conversely, electrical energy is used to bring about certain chemical reactions which are industrially important such as purification of metals like copper, aluminium, generation of gaseous chlorine, oxygen, hydrogen, electroplating, metal coatings etc. The electrochemical or electrolytic processes are carried out in a device known as a cell. An electrolytic (or) electrochemical cell consists of two conducting metal electrodes in contact with an electrolyte solution which separates them (or) placed separately in compartments containing suitable electrolytes. The electrolyte may be an aqueous solution containing mostly the salt of the metal with which the electrode is made of (or) it may be an ionically conducting solid.
There are two types of cells known as
electrolytic cell and electrochemical cell. Each of them possesses different
characteristics and used in different application.
Generally at the anode oxidation reaction
occurs and at the cathode reduction reaction occurs. When the electrodes are
connected externally through a wire and electrons flow through them, the
electrical circuit is said to be an open circuit. If the electrodes are not
connected externally and the electrons do not flow from one electrode into the
other, the electrical circuit is said to be a closed circuit.
Daniel
cell : Daniel cell or a galvanic cell is an example of
electrochemical cell. The overall
reaction taking place in the cell is the redox reaction given as
Zn(s) + Cu2+(aq) -- -- -- > Zn2+(aq) + Cu(s)
This overall reaction is made of the summation
of two half reactions such as oxidation half reaction and reduction half
reaction.
The oxidation half reaction occurring at the
zinc electrode in contact with the aqueous electrolyte containing Zn2+,
accumulates the electrons at the zinc rod.
Zn(s) -- -- -- > Zn2+(aq) + 2e-
The reduction half reaction occurring at the
copper electrode in contact with the aqueous electrolyte containing Cu2+
ions receives the electrons from the zinc electrode when connected externally,
to produce metallic copper according to the reaction as,
Cu2+ + 2e-
-- -- -- > Cu(s)
The decrease in the energy which appears as the
heat energy when a zinc rod is directly dipped into the zinc sulphate solution,
is converted into electrical energy when the same reaction takes place
indirectly in an electrochemical cell. The zinc sulphate is placed in the porous
pot while copper sulphate is placed in a glass vessel.
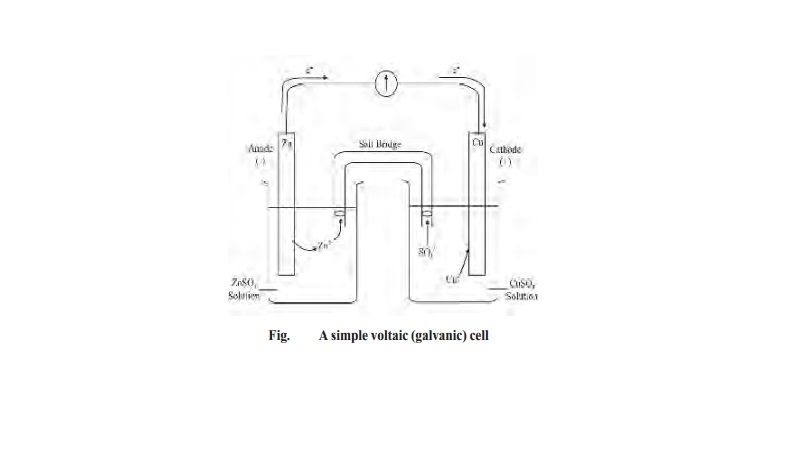
The Daniel cell is also called as the voltaic
cell. However for continuous supply of current for a long period, the two half
cells each comprising the metal electrode and its aqueous electrolyte kept in
separate containers and can be connected externally as below :
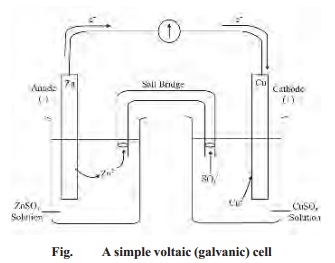
When the cell is set up, electrons flow from
zinc electrode through the wire to the copper cathode. As a result, zinc dissolves
in the anode solution to form Zn2+ ions. The Cu2+ ions in
the cathode half cell pick up electrons and are converted to Cu atoms on the
cathode.
Related Topics