Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Ostwald's theory and Quinonoid Theory
There are two theories to explain the function
of acid-base indicators.
1. Ostwald's theory
This theory was proposed by Ostwald's in 1891.
It is based on Arrhenius theory. According to this theory, the acid-base
indicator is either a weak acid or a weak base. They are partially ionised in
solution. The ionised and unionised forms have different colours. The indicator
exists predominantly in one of the two forms depending on the nature of the
medium and hence there is colour change when the nature of the medium changes.
Phenolphthalein is a weak acid and it is partially ionised in solutions.
HPh (Unionised form (colourless) < -- -- -- > H+ + Ph -
(ionised form (pink) )
In acidic medium, excess H+ ions are
present which suppress the dissociation of HpH due to common ion effect. Hence
the indicator exists predominantly in unionised form and it is colourless. In
alkaline medium, the OH- ion neutralises H+ ion to form
water. Consequently the dissociation of HpH is favoured and the indicator is
predominantly in the ionised form and it is pink in colour.
Methyl orange is a weak base and its ionisation
can be written as
MeOH (Unionised form (yellow)) < -- -- -- > Me+ + OH- (ionised form (pink))
In the presence of a base excess OH-
ions suppress the dissociation of MeOH due to common ion effect. Hence in basic
medium, the indicator is mostly in unionised form which is yellow.
In acidic solution the H+ ions
combine with OH- ions to form unionised water. Hence in acidic
solution, the indicator is mostly in ionised form and has pink colour.
This theory also explains why phenolphthalein
is not a suitable indicator in the titration of a strong acid against a weak
base. The reason is the OH- ions produced by the weak base at the
end point is too low to cause the ionisation of phenolphthalein. Hence, the
pink colour does not appear exactly at the equivalence point. The pink colour
appears only after a sufficient excess of the weak base is added.
For a similar reason, methyl orange is not a
suitable indicator in the titration of a strong base against a weak acid. The
weak acid does not furnish sufficient H+ ions to shift the
equilibrium towards the right. A sufficient excess of the weak acid has to be
added to get the colour change.
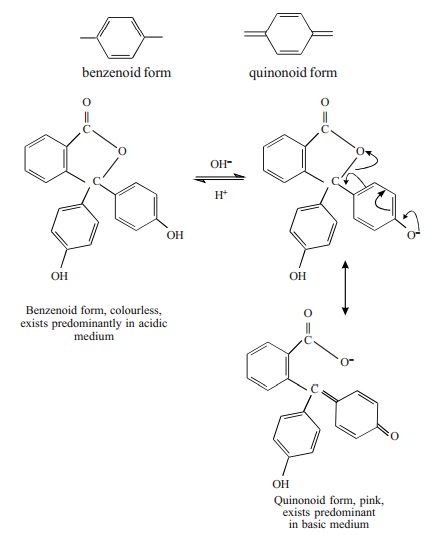
Quinonoid Theory
According to this theory the colour change of
an acid-base indicator arises as a result of structural change. It is supposed
that an indicator exists as an equilibrium mixture of two tautomeric forms
namely, benzenoid and quinonoid forms.
One form exists in acidic solution and the
other form in basic solution. At least one of the tautomers is a weak acid or a
weak base. The two forms possess two different colours and as the pH of the
solution containing the indicator is changed, the solution shows a change of
colour. The colour change is due to the fact that one tautomer changes over to
the other.
For example, phenolphthalein is tautomeric
mixture of the two forms.
Related Topics