Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Difference between chemical reactions and nuclear reactions
Difference between chemical reactions and nuclear
reactions
In ordinary chemical reactions, the nuclei of the atoms
taking part in a chemical reaction remain unaffected. Only the electrons
in the extranuclear part of atoms take part
in the chemical process.
However, during disintegration of atoms (naturally or
artificially), the nuclei of atoms are
affected resulting in the formation of new nuclei. Such reactions in which the nuclei of the atoms interact with other nuclei
or lighter particles or photons resulting
in the formation of new nuclei and one or more lighter particles are called nuclear reactions.
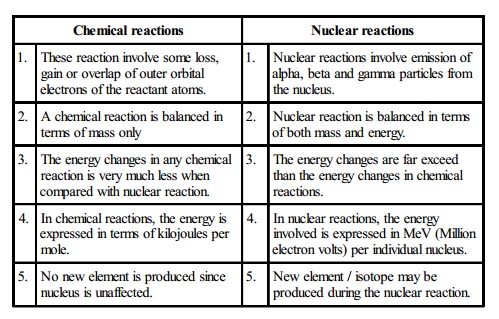
Chemical reactions
1. These
reaction involve some loss, gain or overlap of outer orbital electrons of the
reactant atoms.
2. A
chemical reaction is balanced in terms of mass only
3. The
energy changes in any chemical reaction is very much less when compared with
nuclear reaction.
4. In
chemical reactions, the energy is expressed in terms of kilojoules per mole.
5. No
new element is produced since nucleus is unaffected.
Nuclear reactions
1. Nuclear
reactions involve emission of alpha, beta and gamma particles from the nucleus.
2. Nuclear
reaction is balanced in terms of both mass and energy.
3. The
energy changes are far exceed than the energy changes in chemical reactions.
4. In nuclear reactions, the energy involved is expressed in MeV (Million electron volts) per individual nucleus.
New element / isotope may be produced during the nuclear reaction.
The following facts are taken into account while
expressing a nuclear reaction:
i.
Reactions are
written like a chemical equation. Reactants are written on the left hand side and products on the right hand side with
an arrow in between.
ii.
Mass number is
written as super script on the symbol of the element. For example 7N14 stands
for an atom of nitrogen with mass number 14 and atomic number 7.
iii.
In a chemical
reaction the total number of atoms of various elements are balanced on the two sides. Similarly in nuclear reaction
, the total mass number and atomic
number are balanced on the two sides.
iv.
Symbols used for
projectiles:
The bombarding
particles are called projectiles. These projectiles are represented by the following symbols.
0n1 - neutron
1H1or p
- proton
2H4
- proton
1H2or D2 - proton
1e0 or e - electron or β-particle
+1e0 - positron
The nucleus to be attacked is called as target nucleus
or parent. The new nuclide is called
as recoil nucleus or daughter. The particle ejected during a nuclear reaction is called as ejected particle.
7N14
+ 2He4 -- > 8O17
+ 1H1
This reaction can be represented as (a, p) type
reaction .Hence the above reaction is
represented as
7N14 (a,p) 8O17.
Q value of a nuclear reaction
The amount of energy absorbed or released during nuclear
reaction is called
Q-value of nuclear
reaction.
Qvalue = (mp-mr) 931 MeV
where mr - Sum of
the masses of reactants
mp - Sum of
the masses of products
In the case of energy absorbed then mp>mr, then Q
value will be positive. Q value of a
nuclear reaction in the case of energy released = (mp-mr) 931
MeV. In the case of energy released, mr>mp, and hence Q value will be negative.
Related Topics