Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Difference between Crystalline and Amorphous Solids
Difference between
Crystalline and Amorphous Solids.
Crystalline and amorphous solids differ from one
another in the following respects
1. Characteristic
geometry
A crystalline solid has a definite and regular
geometry due to definite and orderly arrangement of molecules or atoms in
three-dimensional space. An amorphous solid, on the other hand, does not have
any pattern of arrangement of molecules or atoms and, therefore, does not have
any define geometrical shape. It has been found that even if some orderly
arrangement of molecules or atoms exists in a few amorphous solids, it does not
extend more than a few Angstrom units. Thus unlike crystalline solids,
amorphous solids do not have a long range order.
2. Melting points
As a solid is heated, it's molecular vibrations increase and ultimately
becomes so great that molecules break away from their fixed positions. They now
begin to move more freely and have rotational motion as well. The solid now
changes into liquid state. The temperature at which this occurs is known as the
melting point.
A
crystalline substance has a sharp melting point, i.e., it changes abruptly into
liquid state. An amorphous substance, on the contrary, does not have a sharp
melting point. For example, if glass is heated gradually, it softens and starts
to flow without undergoing a definite and abrupt change into liquid state. The
amorphous solids are, therefore, regarded as liquids at all temperatures. There
is some justification for this view because it is known form X-ray examination
that amorphous substance do not have well-ordered molecular or atomic
arrangements. Strictly speaking, solid state refers to crystalline state, i.e.,
only a crystalline material can be considered to be a true solid.
3. Isotropy and
Anisotropy
Amorphous substances differ from crystalline
solids and resemble liquids in another important respect. The properties such
as electrical conductivity thermal conductivity, mechanical strength and refractive
index are the same in all directions. Amorphous substances are, therefore, said
to be isotropic. Liquids and gases are also isotropic. Crystalline solids, on
the other hand, are anisotropic, i.e.,
their physical properties are
different in different directions. For example, the velocity of light passing through a crystal varies with
the direction in which it is measured. Thus, a ray of light entering such a
crystal may split up into two components each following a different path and
travelling with a different velocity. This phenomenon is known as double refraction. Thus, anisotropy in
itself is a strong evidence for the existence of ordered molecular arrangements
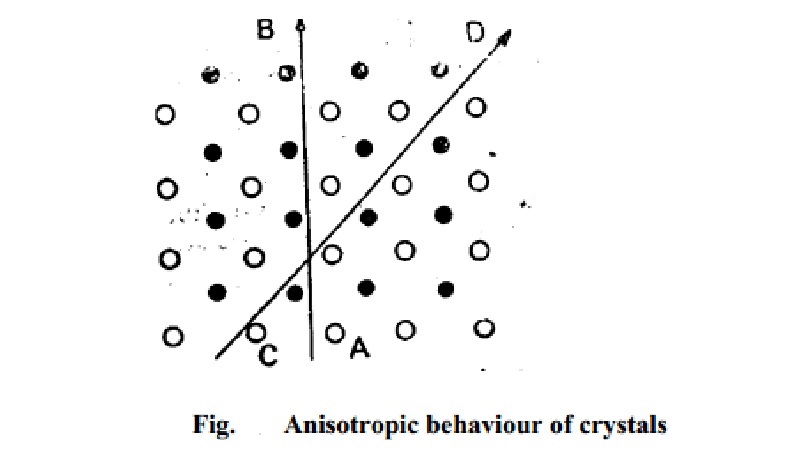
in such materials. This can be shown on reference to Fig. in which a simple
two-dimensional arrangement of only two different kinds of atoms is depicted.
If the properties are measured along the direction indicated by the
slanting line CD, they will be different from those measured in the direction
indicated by the vertical line AB. The reason is that while in the first case,
each row is made up of alternate type of atoms, in the second case, each row is
made up of one type of atoms only. In amorphous solids as well as in liquids
and gases, atoms or molecules are arranged at random and in a disorderly manner
and, therefore, all directions are identical and all properties are alike in
all directions.
Related Topics