Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Crystalline solids and Amorphous solids
Crystalline solids
Some solids, like sodium chloride, sulphur and sugar, besides being
incompressible and rigid, have also characteristic geometrical forms. In these
solids the atoms or molecules are arranged in a very regular and orderly
fashion in a three dimensional pattern. Such substances are called crystalline solid.
The X-ray diffraction studies reveal that their ultimate particles
(viz., molecules, atoms or ions) are arranged in a definite pattern throughout
the entire three-dimensional net-work of a crystal. This definite and ordered
arrangement of molecules, atoms or ions (as the case may be) extends over a
large distance. This is termed as long-range
order.
The outstanding characteristics of a crystalline
solid are its sharp melting point. Crystalline solids are anisotropic since they exhibit different physical properties in all
directions e.g., the electrical and thermal conductivities are different in
different directions.
Amorphous solids
There is
another category of solids such as glass, rubber and plastics, which possess
properties of incompressibility and rigidity to a certain extent but do not
have definite geometrical forms. Such substances are called amorphous solids
Amorphous solids (from the Greek words for 'with
out form') neither have ordered arrangement nor sharp melting point like
crystals but when heated, they become pliable until they assume the properties
usually related to liquids. These solids lack well-defined faces and shapes.
Many amorphous solids are mixture of molecules that do not stick together well.
Most others are composed of large complicated molecules. Amorphous solids are
therefore regarded as super cooled liquids with high material becomes rigid but
there the forces of attraction holding the molecules together are so great that
the material becomes rigid but there is no regularity of structure. Thus,
amorphous solids do not melt at specific temperatures. Instead they soften over
a temperature range as intermolecular forces of various strengths are overcome.
Amorphous solids are isotropic as they exhibit same physical properties in all the
directions.
Difference
between Crystalline and Amorphous Solids. Crystalline and amorphous solids differ from one
another in the following respects
1. Characteristic
geometry
A crystalline solid has a definite and regular
geometry due to definite and orderly arrangement of molecules or atoms in
three-dimensional space. An amorphous solid, on the other hand, does not have
any pattern of arrangement of molecules or atoms and, therefore, does not have
any define geometrical shape. It has been found that even if some orderly
arrangement of molecules or atoms exists in a few amorphous solids, it does not
extend more than a few Angstrom units. Thus unlike crystalline solids,
amorphous solids do not have a long range order.
2. Melting points
As a solid is heated, it's molecular vibrations increase and ultimately
becomes so great that molecules break away from their fixed positions. They now
begin to move more freely and have rotational motion as well. The solid now
changes into liquid state. The temperature at which this occurs is known as the
melting point.
A
crystalline substance has a sharp melting point, i.e., it changes abruptly into
liquid state. An amorphous substance, on the contrary, does not have a sharp
melting point. For example, if glass is heated gradually, it softens and starts
to flow without undergoing a definite and abrupt change into liquid state. The
amorphous solids are, therefore, regarded as liquids at all temperatures. There
is some justification for this view because it is known form X-ray examination
that amorphous substance do not have well-ordered molecular or atomic
arrangements. Strictly speaking, solid state refers to crystalline state, i.e.,
only a crystalline material can be considered to be a true solid.
3. Isotropy and
Anisotropy
Amorphous substances differ from crystalline
solids and resemble liquids in another important respect. The properties such
as electrical conductivity thermal conductivity, mechanical strength and refractive
index are the same in all directions. Amorphous substances are, therefore, said
to be isotropic. Liquids and gases are also isotropic. Crystalline solids, on
the other hand, are anisotropic, i.e.,
their physical properties are
different in different directions. For example, the velocity of light passing through a crystal varies with
the direction in which it is measured. Thus, a ray of light entering such a
crystal may split up into two components each following a different path and
travelling with a different velocity. This phenomenon is known as double refraction. Thus, anisotropy in
itself is a strong evidence for the existence of ordered molecular arrangements
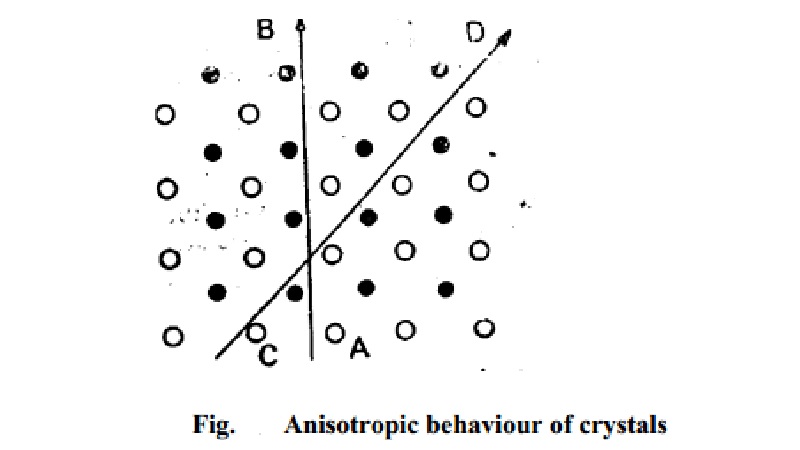
in such materials. This can be shown on reference to Fig. in which a simple
two-dimensional arrangement of only two different kinds of atoms is depicted.
If the properties are measured along the direction indicated by the
slanting line CD, they will be different from those measured in the direction
indicated by the vertical line AB. The reason is that while in the first case,
each row is made up of alternate type of atoms, in the second case, each row is
made up of one type of atoms only. In amorphous solids as well as in liquids
and gases, atoms or molecules are arranged at random and in a disorderly manner
and, therefore, all directions are identical and all properties are alike in
all directions.
Size and shape of crystals
Several naturally occurring solids have definite crystalline shapes,
which can be recognized easily. There are many other solid materials, which
occur as powders or agglomerates of fine particles and appear to be amorphous.
But when an individual particle is examined under a microscope, it is also seen
to have a definite crystalline shape. Such solids, in which the crystals are so
small that can be recognized only under a powerful microscope, are said to be microcrystalline. The size of a crystal
depends on the rate at which it is formed: the slower the rate the bigger the
crystal. This is because time is needed by the atoms or molecules to find their
proper positions in the crystal structure. Thus, large transparent crystals of
sodium chloride, silver chloride, lithium chloride, etc., can be prepared by
melting these salts and allowing them to cool very slowly at a uniform rate. It
is for this reason that crystals of most of the minerals formed by geological
processes are often very large.
Crystal
possess the following characteristic feature:
i)Faces:
Crystals are bound by plane faces. The surfaces usually plannar and arranged on
a definite plane (as a result of internal geometry), which bind crystals are
called faces.
Faces are
of two types:
Like: A crystal having all faces alike e.g. Fluorspar.
Unlike: A
crystal having all faces not alike e.g. Galena.
ii) Form: All the faces corresponding to a crystal are said to
constitute a form.
iii) Edges: The intersection of two adjacent faces gives rise to the
formation of edge.
iv) Interfacial Angle: The angle between the normals to the two
intersecting faces is called interfacial angle.
Although the size of the faces or even faces of the crystals of the same
substance may vary widely with conditions of formation, etc., yet the
interfacial angles for any two corresponding faces of the crystals remain
invariably the same throughout.
Although
the external shape is different yet the interfacial angles are same. The
measurement of interfacial angles in crystals is, therefore, important in the
study of crystals. The subject is known as crystallography.
Related Topics