Chapter: The Massage Connection ANATOMY AND PHYSIOLOGY : Introduction to Anatomy and Physiology
Chemical Level of Organization
CHEMICAL LEVEL OF ORGANIZATION
Although a therapist seems to work at the systemic level, the benefits of therapy are a result of changes produced at the chemical and cellular levels. Diseases, although they produce symptoms such as pain, fever, and edema, are actually caused by dysfunction at the cellular and chemical levels. Small changes in chemi-cal makeup can have serious effects. For example, in a disease known as sickle cell anemia, the protein in the hemoglobin molecule is slightly different from normal. This small change has drastic effects on the properties of hemoglobin. The hemoglobin in sickle cell anemia, unlike normal hemoglobin, changes into a more solid form in an environment with less oxygen,resulting in red cells that become sickle-shaped and break up easily. The final outcome—less red cells, less oxygen available to the cells—the patient has difficulty performing normal, day-to-day activities. All because of a slight change in the protein structure in hemo-globin.
Water, which makes up 50–60% of body weight, has properties that are used to cool the body by evap-oration. Water is the medium in which ions dissolve and cells float. The acidity and alkalinity of this medium determine how well the various chemical re-actions of the body occur. Therapists use water prop-erties to their advantage. Water is used for heat and cold application. During rehabilitation, water exer-cise has been found to be beneficial. Knowledge of the body at the chemical level and the chemical prop- erties of water and other common substances are, therefore, beneficial to therapists.
The Atom
At the chemical level, the smallest unit of matter is the atom (see Figure 1.10). All living and nonliving thingsare made up of atoms. The characteristics of the each substance result from the types of atoms involved and how they are combined. An atom is made up of three different types of particles—protons, neutrons, and electrons.Protons carry a positive (+) electricalcharge; neutrons have no charge; and electrons carry a negative (-) charge. The protons and neutrons are al-most the same size and mass, while the electrons are much lighter. Hence, the weight of the body is equal to all the neutrons and protons combined.
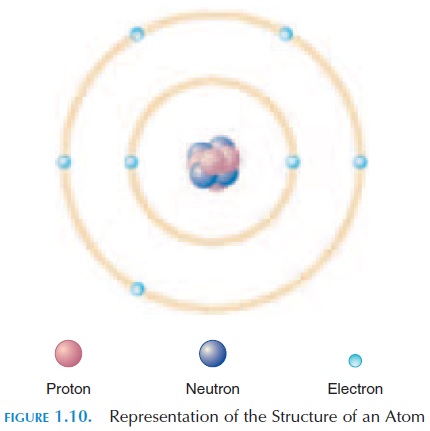
Normally, because positive charges attract nega-tive charges, an atom carries an equal number of pro-tons and electrons. The number of protons in an atom is known as theatomic number. For example, a hydrogen atom has an atomic number of 1, mean-ing that it has one proton and one electron. The pos-itively charged proton is usually in the center, with the nucleus and the negatively charged electron moving around it in an orbit referred to as the elec-tron shell.
At times, the electrons may not equal the number of protons in an atom. In this case, the chemical may have more positive charges or more negative charges. They then tend to attract or repel other chemicals, depending on their charges. This is the basis for the movement of an electrical impulse down the nerves.
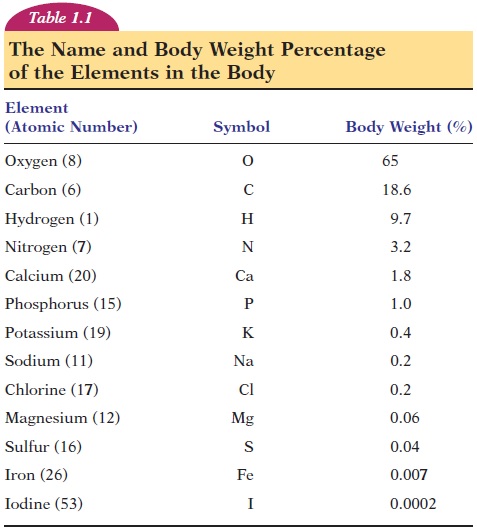
All atoms are assigned to groups called elements, based on the number of protons they carry or, in other words, on their atomic number. Elements are substances that cannot be split into simpler sub-stances by ordinary chemical means. (An atom is the smallest unit of matter that has the properties and characteristics of elements.) There are 92 elements in nature. As a standard, each element is given a sym-bol. Many symbols are connected to their Englishnames, while other symbols to their Latin names. The symbols and percentage of body weight for thir-teen of the most abundant elements in the human body are given in Table 1.1. These elements are mostly combined with other elements (discussed later). Note that the elements oxygen, carbon, hydro-gen, and nitrogen contribute to more than 90% of body weight. In addition to these elements, the body has minute quantities of other elements (trace ele-ments), such as silicon, fluorine, copper, manganese,zinc, selenium, and cobalt.
Isotopes
The atoms of the same element may have different numbers of neutrons in the nucleus. Although this difference in number does not affect the property of the atom, the weight of the atoms may differ (Re-member, neutrons are of the same mass and size as protons.). Hydrogen, for example, may have a proton and no neutrons or one neutron or two neutrons in the nucleus. The atoms of an element that has a dif-ferent number of neutrons in the nucleus are known as isotopes. Isotopes are referred to by the combined number of protons and neutrons (i.e., mass num-ber).
The mass number is the number of protons andneutrons in an atom. In the above example, hydrogen with one proton is hydrogen-1 (1H); with one proton and one neutron, hydrogen-2 (2H); and with one pro-ton and two neutrons, hydrogen-3 (3H).
Some of the isotopes of certain elements contain nuclei, which spontaneously emit subatomic parti-cles known as radioisotopes. Radioisotopes are said to be radioactive. These emissions can be dangerous as they can damage or destroy cells and exposure to these emissions increases the risk of cancer. In medi-cine, radioisotopes are used for medical imaging and destroying cancerous cells.
Electrons, Energy Levels, and Chemical Bonds
Generally, atoms have the same number of protons and electrons (an equal number of positive and nega-tive charges) and are considered electrically neutral.
Even when the protons and electrons in an atom are equal, not all electrons can orbit in the same elec-tron shell. Each electron shell can hold only a specific number of electrons. For example, the first shell (closest to the nucleus) can hold two electrons; the second shell, eight electrons; the third shell, eight electrons; the fourth shell, 18 electrons, and so on. Imagine a circular theatre, with a stage in the center and chairs arranged in successive circular rows. Not all of the audience can sit in the first row. If there are only a few people, even the first row may not get filled. If there are more people in the audience, the first row gets filled and may spill over to the second row. Depending on the number of people, the second row may or may not get filled. Similarly, there are many electron shells (referred to here as energy lev-els) in an atom. The first energy level can have onlytwo electrons in its orbit, and the second level can have only eight. The number of electrons in the en-ergy level affects the property of the atom. Atoms with energy levels that are not full react with other atoms and try to fill the level. For example, the hy-drogen atom has an atomic number of 1 (one proton and one electron). Because the first energy level is lacking one electron, it tries to attract another elec- tron to fill the level. In this way, atoms with outer en-ergy levels that are not full gain, loose, or share elec-trons to fill the outer energy level. This interaction in-volves the formation of chemical bonds that hold the interacting atoms together, maintaining stability. Atoms with full outer shells are stable—they do not take part in these reactions—and are said to be inert.
When atoms are held together by chemical bonds, the property of this “particle” is different from that of the individual atoms. Water, for example, forms by bonding two hydrogen atoms and one oxygen atom. The product (water) has completely different proper-ties than hydrogen or oxygen. A chemical structure formed with two or more elements is referred to as a compound—a substance that can be broken downinto its elements by ordinary chemical means. When atoms held together by bonds share electrons, the re-sulting substance is called a molecule. A molecule may have atoms of the same element or of different elements.
When atoms are bonded together, the resulting substance is denoted by a molecular formula. The formula indicates the involved elements by their chemical symbol. The number of each element in-volved in forming the molecule or compound is de-noted, in subscript, beside the element. For example, water is made up of two hydrogen atoms and one oxy-gen atom. The molecular formula for water is H2O.
Chemical Bonds
Atoms can interact in three ways; therefore, there are three types of chemical bonds—ionic bonds, cova-lent bonds, and hydrogen bonds.
Ionic Bonds
Some atoms may lose or gain an electron when bond-ing with another atom. In the first case, this atom has one electron less than the number of protons, mean-ing that there are more positive than negative charges. This atom is referred to as a cation (posi-tively charged). In the second case, this atom gains an electron and has more electrons than protons, meaning that there are more negative charges than positive charges. This atom is referred to as an an-ion. Both cations and anions, with their unequalnumber of protons and electrons, are known as ions. Ions are denoted by their chemical symbol, with pos-itive or negative signs given in superscript. For ex-ample, sodium ion is represented as Na ; chlorine as Cl-. Being positively charged, cations attract anions and vice versa. This type of bonding is known as ionic bonds. Ionic bonds and ions are especially im-portant in nerve conduction and brain activity.
The formation of salt—table salt is a good example (see Figure 1.11). The chemical name of table salt is sodium chloride (NaCl); it is made up of sodium and chlorine. Sodium has an atomic number of 11. This means that it has 11 protons (and 11 electrons) to be neutral. Of the eleven electrons, two occupy the first energy level and eight occupy the second energy level and fill it. The remaining one electron (2 8 10) or-bits alone in the third energy level. Because the outer energy level is not full, the sodium atom is reactive and tends to donate its electron to another atom.
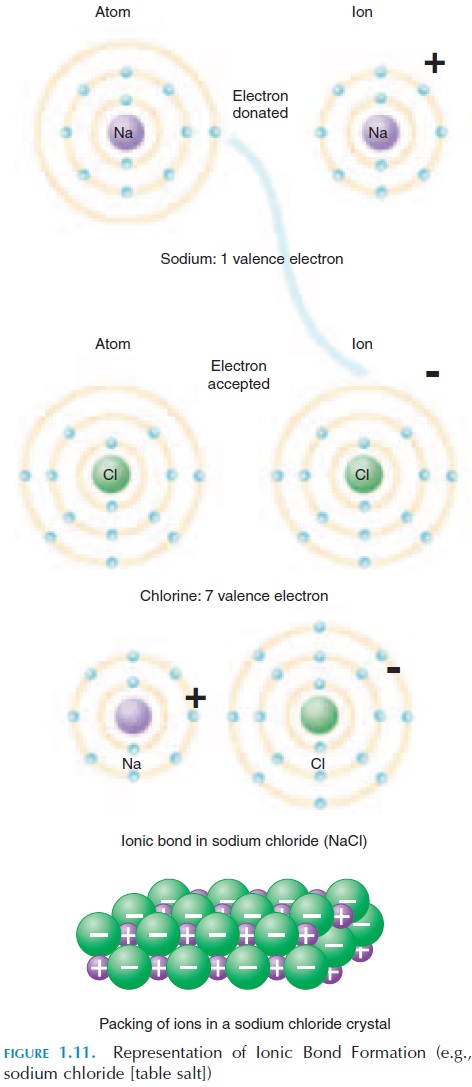
Chlorine has an atomic number of 17. This means it has 17 protons and 17 electrons, making it neutral. Of the electrons, two occupy the first energy level, eight occupy the second level, and seven occupy the outer level. One more electron will fill its outer energy level; chlorine has a tendency to attract an electron.
By ionic bond, sodium and chlorine come together to satisfy each other’s needs. Sharing electrons, the positively charged sodium is attracted to the nega-tively charged chlorine and they stay together to form the compound sodium chloride—table salt. When sodium chloride is dissolved in water, the ions sepa-rate—ionize—and positively charged sodium ions (Na ) and negatively charged chloride ions (Cl-) are found.
Covalent Bonds
In some cases, atoms share their electrons rather than gain or lose them. Such bonds are known as co-valent bonds (see Figure 1.12). The resultant chemi-cal is termed amolecule. A good example is the ele-ment hydrogen. Hydrogen has an atomic number of 1 (i.e., 1 proton and 1 electron). However, the outer
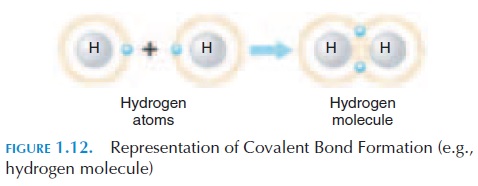
shell needs another electron to be complete. There-fore, one hydrogen atom shares its electron with an-other hydrogen atom, completing their energy levels and forming a molecule. This is how hydrogen nor-mally exists—in pairs, and is referred to as hydrogen molecules. A hydrogen molecule is symbolized as H2.
Similarly, many different elements may bond to-gether. Carbon dioxide gas has one carbon atom and two oxygen atoms bonded covalently (CO2). Water has two hydrogen atoms and one oxygen atom bonded covalently (H2O). Elements may share one, two, or three electrons. In the human body, covalent bonds are the most common.
When covalent bonds are formed, the electrons may be shared equally or unequally between the specific atoms. When shared equally, these bonds are known as nonpolar covalent bonds. Sometimes, one atom at-tracts the shared electron more than the other atom. In this case, the atom that attracts the electron to a greater degree would be slightly more negatively charged than the other atom. The other atom will be slightly more positively charged. These charges are represented with the symbol or - (see Figure 1.13). These covalent bonds are known as polar covalent bonds.
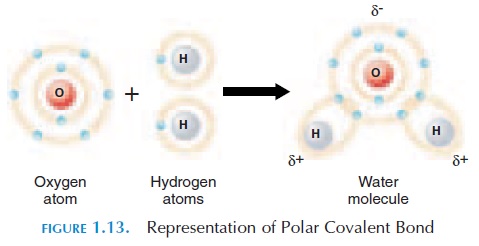
Hydrogen Bonds
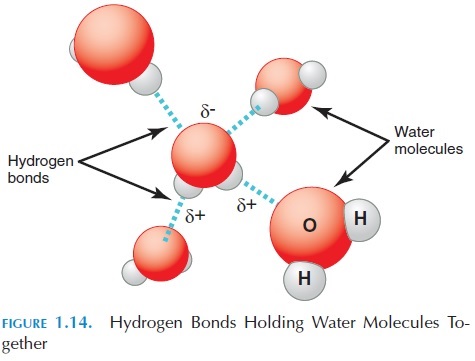
Other than ionic and covalent bonds, other weak at-tractions may be present between atoms of the same molecule or compound or between atoms in other molecules. The most important of these weak attrac-tions are hydrogen bonds, in which a hydrogen atom involved in a polar covalent bond is attracted to oxy-gen or nitrogen involved in a polar covalent bond by itself. This attraction is important. Although mole-cules are not formed through the hydrogen bonds, this bonding can alter the shape of the molecules. For example, it is this weak attraction that holds water together and makes it form a drop. We refer to this force as surface tension (see Figure 1.14). At the molecular level in the body, these hydrogen bonds can alter the properties of proteins, making them change their shape and structure.
Matter exists as solids, liquids, or gases as a result of the degree of interaction between the atoms and molecules. For example, hydrogen molecules do not attract each other and, therefore, exist as gas. Water, however, has more interactions and exists as liquid throughout a wide temperature range.
Chemical Reactions
There is a constant reaction in the human body be-tween atoms and molecules. Cells control these reac-tions to stay alive. In the chemical reaction, new bonds form between atoms or present bonds break down to form a different compound. The term me-tabolism refers to all the chemical reactions that oc-cur in the body. When a chemical reaction occurs, en-ergy may be expended or released.
What is energy? Energy is the capacity to work, and work is movement or a change in the physical struc-ture of matter. Energy can be in two forms—potentialenergy orkinetic energy. For example, imagine anelastic band stretched across two poles. The stretched elastic band has potential energy. If the band comes undone from one pole, it springs back to its original length.
This is kinetic energy. Of course, kinetic energy was initially used to stretch the elastic band and tie it to the two poles. Remember that energy cannot be lost, it is only converted from one form to another.
During chemical reactions, much of the energy in the body is converted to heat, which maintains the core body temperature. When the body is cold, me-tabolism (chemical reactions) increases and more heat is produced. That’s why we shiver. The muscles quickly contract and relax (shivering), and the chem-ical reactions that occur during this process generate the needed heat.
The body “captures” energy in the form of high-energy compounds. These compounds require energy to build up; however, when broken down, they release a lot of energy. This high-energy compound is adeno-sine triphosphate (ATP). ATP is formed from thechemicals adenosine monophosphate (AMP) and adenosine diphosphate (ADP) by combining withphosphorus. For example, chemical reactions in the body break glucose down into smaller compounds. The energy that is released is “captured” by combin-ing ADP with organic phosphate to form ATP. When mechanical energy is needed to walk, ATP is broken down to release energy and ADP and P.
Types of Chemical Reactions in the Body
Many types of reactions take place in the body. Some reactions occur to break down compounds into smaller bits. This is a decomposition reaction.
AB → A + B
This is what happens when food is broken down and digested. Similarly, when a person loses weight, fat is broken down into smaller fragments. Within the cell, chemical reactions break down substances and the energy released is used to do work. This process is known as catabolism.
Building up, or synthesis, is the opposite of decom-position. In this process, kinetic energy is invariably used to form compounds from fragments. The kinetic energy is converted to potential energy to be used later in a decomposition reaction when work is needed. The process of building up is known as anabolism.
A B → AB
Another reaction that occurs in the body is ex-change. In this process, the fragments get shuffled.
AB+CD → AD+CB
Some reactions can proceed in both ways. The di-rection in which the reaction proceeds is altered by many factors, referred to as reversible reactions.
AB → A+B → AB
Reversible reactions may be represented as:
AB ↔ A+B
The Role of Enzymes
The various chemical reactions in the body would proceed too slowly to be of any use if they did not have mechanisms in place to speed up the reaction. The enzyme is one of the mechanisms that help that process. Enzymes are proteins and, although they do not actually participate in the chemical reaction it-self, facilitate the reaction. Enzymes do not get con-sumed or altered in the process. The body has nu-merous enzymes that speed up specific chemical reactions. The importance of specific enzymes is re-alized when one of them is deficient in the body.
Enzyme activity can be modified by various factors, such as temperature, acidity, or alkalinity. For exam-ple, the activity of many enzymes is significantly re-duced when the temperature drops, slowing down chemical reactions. Similarly, an acidic environment is detrimental to enzymes. When muscle activity is in-creased, many chemical reactions are triggered to produce energy for contraction. One of the metabo-lites formed, especially if oxygen supply is inadequate, is lactic acid. If this metabolite is not rapidly removed, the muscle environment becomes acidic and the ac- tivity of various enzymes slows down or stops and muscle fatigue results.
Acidity and Alkalinity
For the purpose of enzyme activity and to maintain the shape and structure of the proteins, the body must maintain the right state of acidity and alkalin-ity. If there are more H+(hydrogen ions), a solution is acidic. If there are more OH- (hydroxyl ions), the so-lution is alkaline. The acidity and alkalinity of a solu-tion is measured in terms of pH(hydrogen ion con-centration). As the quantity of hydrogen ion is so small, it is cumbersome to express in actual numbers. If needed, the number would be something like 0.0000001. To make it easier, this is expressed by pH. The pH is actually a measure of hydrogen ion con-centration in the body fluid; the pH scale extends from 0 to 14. Water is considered to be a pH of 7.0; a neutral pH. This means that water contains 0.0000001, or 1 Π10-7 of a mole of hydrogen ions per liter. If the pH is lower than 7.0, it denotes that the fluid has more hydrogen ions or that it is acidic. For example, if a solution has a pH of 5.0, it contains 0.00001 or 1 Π10-5 of a mole of hydrogen ions per liter (i.e., more hydrogen ions than a solution of pH 7.0). If a solution has a pH above 7.0, it has less hy-drogen ions than water and is alkaline.
The pH of the body is 7.4 (range, 7.35–7.45) (i.e., slightly alkaline). For body enzymes to be active and for chemical reactions to proceed optimally, it is vital that pH be maintained at this level. This implies that the body needs regulatory mechanisms that monitor the hydrogen ion levels carefully and get rid of them as and when they form above normal levels.
One of the body’s compensatory mechanisms is the presence of many buffers. Buffers are compounds that prevent the hydrogen ion concentration from fluctuating too much and too rapidly to alter the pH. The body uses buffers to convert strong acids (that dissociate easily into hydrogen ions) to weak acids (that dissociate less easily). Proteins, hemoglobin, and a combination of bicarbonate and carbonic acid compounds are a few of the buffers present in body fluids. The later is an important buffer. The following chemical reaction indicates how a combination of bi-carbonate and carbonic acid compounds work as buffers.
HCO3- H → H2CO3 → H2O CO2
In this chemical reaction, HCO3 (bicarbonate), a weak base, combines with the hydrogen ions to form H2CO3 (carbonic acid), a weak acid. This weak acid can be further broken down to CO2 (carbon dioxide), which can be breathed out, and H2O (water), which can be used for other reactions or excreted by the kid-neys. Alternately, if the pH becomes acidic, the weak carbonic acid H2CO3 can break down to form HCO3-(a weak base) and H+(hydrogen ions).
Important Organic Compounds in the Body
Organic compounds are compounds that have the el-ements carbon, hydrogen and, usually, oxygen. Many of the compounds have the carbon atoms in chains, linked by covalent bonds. There are four important organic compounds in the body—carbohydrates;proteins; fats or lipids; and nucleic acids. The firstthree are vital sources of energy in the body. The structure of the body is mostly made up of proteins. Lipids are needed for building certain structures, such as cell membranes. Lipids are also stored and used as a reserve. Nucleic acids are used to form ge-netic material.
Carbohydrates
Carbohydrates are organic compounds that have car-bon, hydrogen, and oxygen in a ratio of 1:2:1. Sugars and starches are typical examples. Carbohydrates are typical sources of energy for the cell and can be eas- ily broken down by the cells of the body. Carbohy-drates may be simple or complex.
Simple sugars or monosaccharides contain 2–7 carbon atoms. Glucose, for example, has six carbon atoms. Fructose, found in fruits, is another example. Simple sugars dissolve easily in water and are easily transported in the blood. Complex sugars are formed by the combination of two or more simple sugars. They are broken down by the digestive tract into its simplest form before being absorbed into the body.
Simple sugars that are absorbed are reconverted by chemical reactions in the presence of enzymes into various complex forms by the liver, muscle, and other tissues. Glycogen is one form of complex car-bohydrate. This form of carbohydrate is insoluble in body fluids. When the demand for energy goes up, glycogen is broken down into its simple form and transported by blood to the needed areas.
Lipids
Lipids are organic compounds that have carbon, hy-drogen, and oxygen atoms, however, in a different ra-tio than carbohydrates. These compounds are insolu-ble in water and must be transported in the blood by special mechanisms. For example, in blood, lipids combine with proteins and are carried as lipopro-teins. Lipids are used to form important structures,such as cell membranes and certain hormones, and are an important source of energy. When there is more lipid supply than needed, it is stored in various regions for future use. The properties of lipids make them important body insulators. Fatty acids, glyc-erides, steroids, and phospholipids are some of the important lipids found in the body.
Proteins
Proteins are the organic compounds that are most abundant in the body. All proteins contain carbon, hydrogen, oxygen, and nitrogen. In addition, some proteins may contain sulfur. There are about 100,000 different kinds of proteins in the body.
Proteins form the structural framework for the body. The bulk of our muscles are made of proteins.
The enzymes that facilitate chemical reactions are proteins, as are the buffers. The blood contains pro-tein in the plasma. Hemoglobin is a plasma protein used to transport gases. The antibodies are plasma proteins used in defense. Many of the hormones are made of proteins.
Proteins consist of organic molecules chains known as amino acids. There are about 20 significant amino acids in the human body. Different proteins in the body are formed by combining amino acids, using covalent bonds in different sequences. A protein chain may have any number of amino acids and is known as a polypep-tide. Some proteins may have 100,000 or more aminoacids. Each amino acid has a different chemical struc-ture and this, in turn, alters its properties.
The shape of a protein is one property that may be altered. Certain proteins may be flat and appear as a long chain; certain proteins are more complex and form spirals. Others may be folded or coiled to form complex three-dimensional structures (e.g., hemoglo-bin). The structural properties are determined by the sequence in which the amino acids are arranged. The alteration of just one amino acid sequence can alter the function of the protein drastically, as in the ex-ample of sickle cell anemia given earlier.
Nucleic Acids
Nucleic acids are large organic molecules containing carbon, hydrogen, oxygen, nitrogen, and phosphorus. Found in the nucleus of the cell, they are important for storing and processing information in every cell. Nucleic acid is the major component of ova and sperm and conveys such information as shape, eye color, and sex. There are two types of nucleic acid—DNA (deoxyribonucleic acid) and RNA (ribonu-cleic acid).
DNA is in the form of a double helix (i.e., two spi-rals, parallel to each other). The two strands are held together by hydrogen bonds. A small segment of DNA molecule forms a gene. Each gene deter-mines the traits we inherit from our parents. They also control protein synthesis in each cell. The RNA conveys the message from the gene to the cell and determines the amino acids sequence when proteins are synthesized.
High-Energy Compounds
All cells require energy to carry out their functions. This energy is derived by catabolism of organic sub-stances in the presence of enzymes. The energy liber-ated is stored as potential energy in the form of high-energy bonds. High-energy bonds are covalentbonds created in specific organic substrates in the presence of enzymes. When the cell needs energy, these bonds are broken and the energy harnessed.
AMP is the most important organic substrate used by the cells to form covalent bonds. The cells use the energy liberated by nutrient breakdown to convert AMP to ADP, which is then converted to ATP. Both ADP and ATP are formed by attaching phosphate groups by covalent bonds.
ADP + phosphate group + energy → ATP + H2O
ATP → ADP + phosphate group + energy
As the body needs energy, ATP is broken down. Other compounds with high-energy bonds exist, but ATP is the most abundant.
Related Topics