Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Anomalous periodic properties in terms of screening constant, stability etc.
Anomalous periodic
properties in terms of screening constant, stability etc.
According to Hund's rule atoms having half-filled or completely filled
orbitals are comparatively more stable and hence more energy is needed to
remove an electron from such atoms. The ionization potentials of such atoms
are, therefore, relatively higher than expected normally from their position in
the periodic table.
Example
A few irregularities that are seen in the increasing values of
ionization potential along a period can be explained on the basis of the
concept of half-filled and completely filled orbitals, e.g., Be and N in the
second period and Mg and P in the third period have slightly higher values of
ionization potentials than those normally expected. This is explained on the
basis of extra stability of the completely-filled 2s-orbital in Be(Be®2s2) and 3s-orbital in Mg (Mg®3s2) and of half-filled 2p-orbital in
N (N®2s2p6)
and 3p-orbital in P (P®3s2p3).
Another example for irregularity in Ionization
potential is observed in the case of B and Be.
Ionization energy of boron (B ®2s22p1) is lower than that
of beryllium (Be ® 2s2)
[B = 8.3 eV, Be = 9.3 eV], since in case of boron we have to remove a 2p1
electron to get B+[B (2s2p1) ® B+(2S2) + e-]
while in case of Be we have to remove a 2s1 electron of the same
main energy level to have Be+ ion. [Be (2s2) ® Be+(2s1) +e-].
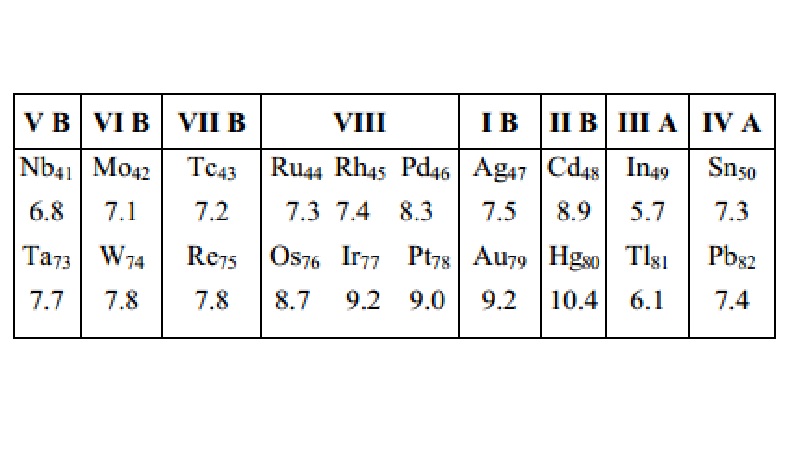
There is an exception to the vertical trend of ionization potential.
This exception occurs in the case of those elements whose atomic numbers are
greater than 72. Thus the ionization potentials of the elements from Ta73
to Pb 82 are greater than those of the elements of the same
sub-group above them as shown below : (First ionization potential values are
given in electron volts, eV).
The reason for the abnormal behaviour (i.e. an
increase in the value of I1 from Nb ® Ta, Mo ® W, …..,Sn
® Pb) shown
by the elements from Ta73 to Pb82 is due to the lanthanide
contraction as a result of which there occurs an increase in the nuclear charge
without a corresponding increase in size through the rare earths. In fact, the
size actually decreases in this region.
Periodic Variations
Similarly in moving down a group electron affinity values generally
decrease, e.g. ECl>EBr>EI. This is due
to the steady increase in the atomic radius of the elements.
Exceptions
There are, however, some exceptions to this
general rule as is evident from the following examples:
It is known that EF < ECl
(EF = 322 kJ mol-1 , ECl = 349 kJ mol-1).
The lower value of E for F is probably due to the electron-electron repulsion
in relatively compact 2p-orbital of
F-atom.
In period, electron affinity values generally increase on moving from left to right in a period in the
periodic table.
Exceptions
There are, however, exceptions also to this general rule; e.g.
Be and Mg have their EA values equal to zero.
Since Be and Mg have completely filled s-orbitals
(Be ® 2s2,
Mg ® 3s2),
the additional electron will be entering the 2p-orbital in case of Be and 3p-orbital
in case of Mg which are of considerably higher energy than the 2s-and 3s orbitals respectively.
Related Topics