Chapter: Biochemistry: The Three-Dimensional Structure of Proteins
Quaternary Structure of Proteins
Quaternary Structure of Proteins
Quaternary
structure is the final level of protein structure and pertains to proteins that
consist of more than one polypeptide chain. Each chain is called a subunit. The number of chains can range
from two to more than a dozen, and the chains may be identical or different.
Commonly occurring examples are dimers,
trimers, and tetramers, consisting
of two, three, and four polypeptidechains, respectively. (The generic term for
such a molecule, made up of a small number of subunits, is oligomer.) The chains interact with one another noncovalently via
electrostatic attractions, hydrogen bonds, and hydrophobic interactions.
As a
result of these noncovalent interactions, subtle changes in structure at one
site on a protein molecule may cause drastic changes in properties at a distant
site. Proteins that exhibit this property are called allosteric. Not all multisubunit proteins exhibit allosteric
effects, but many do.
A
classic illustration of the quaternary structure and its effect on protein
properties is a comparison of hemoglobin, an allosteric protein, with
myoglo-bin, which consists of a single polypeptide chain.
Hemoglobin
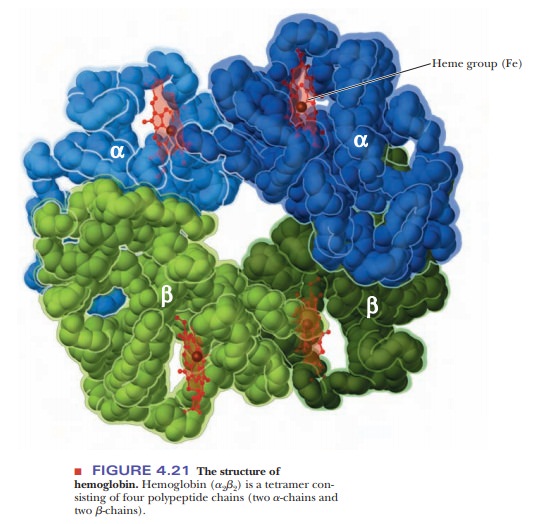
Hemoglobin
is a tetramer, consisting of four polypeptide chains, two α-chains, and two β-chains
(Figure 4.21). (In oligomeric proteins, the types of polypeptide chains are
designated with Greek letters. In this case, the terms a and b have nothing to
do with the α-helix and the β-pleated sheet; rather they just refer to two
different polypeptide chain subunits.) The two α-chains of hemoglobin are
identical, as are the two β-chains. The overall structure of hemoglobin is a2b2 in
Greek-letter notation. Both the α- and β-chains of hemoglobin are very similar
to the myoglobin chain. The α-chain is 141 residues long, and the β-chain is
146 residues long; for comparison, the myoglobin chain is 153 residues long.
Many of the amino acids of the α-chain, the β-chain, and myoglobin are homologous; that is, the same amino acid
residues are in the same positions. The heme group is the same in myoglobin and
hemoglobin.
We have
already seen that one molecule of myoglobin binds one oxygen molecule. Four
molecules of oxygen can therefore bind to one hemoglobin molecule. Both
hemoglobin and myoglobin bind oxygen reversibly, but the binding of oxygen to
hemoglobin exhibits positive
cooperativity, whereas oxy-gen binding to myoglobin does not. Positive
cooperativity means that when one oxygen molecule is bound, it becomes easier
for the next to bind. A graph of the oxygen-binding properties of hemoglobin
and myoglobin is one of the best ways to illustrate this point (Figure 4.22).
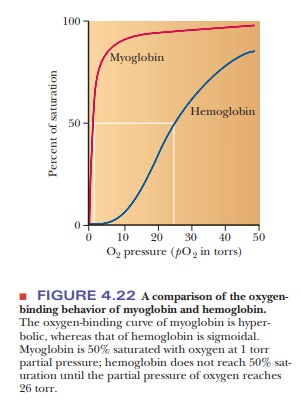
When the
degree of saturation of myoglobin with oxygen is plotted against oxygen
pressure, a steady rise is observed until complete saturation is approached and
the curve levels off. The oxygen-binding curve of myoglobin is thus said to be hyperbolic. In contrast, the shape of
the oxygen-binding curve for hemoglobin is sigmoidal.
This shape indicates that the binding of the first oxygen molecule facilitates
the binding of the second oxygen, which facilitates the binding of the third,
which in turn facilitates the binding of the fourth. This is precisely what is
meant by the term cooperative binding.
However, note that even though cooperative binding means that binding of each
subsequent oxygen is easier than the previous one, the binding curve is still
lower than that of myoglobin at any oxygen pressure. In other words, at any
oxygen pressure, myoglobin will have a higher percentage of saturation than
hemoglobin.
How does hemoglobin work?
The two
different types of behavior exhibited by myoglobin and hemoglobin are related
to the functions of these proteins. Myoglobin has the function of oxygen storage in muscle. It must bind strongly
to oxygen at very low pressures, and it is 50% saturated at 1 torr partial
pressure of oxygen. (The torr is a
widely used unit of pressure, but it is not an SI unit. One torr is the
pressure exerted by a column of mercury 1 mm high at 0°C. One atmosphere is
equal to 760 torr.)
The
function of hemoglobin is oxygen transport,
and it must be able both to bind strongly to oxygen and to release oxygen
easily, depending on conditions. In the alveoli of lungs (where hemoglobin must
bind oxygen for transport to the tissues), the oxygen pressure is 100 torr. At
this pressure, hemoglobin is 100% saturated with oxygen. In the capillaries of
active muscles, the pressure of oxygen is 20 torr, corresponding to less than
50% saturation of hemoglobin, which occurs at 26 torr. In other words,
hemoglobin gives up oxygen easily in capillaries, where the need for oxygen is
great.
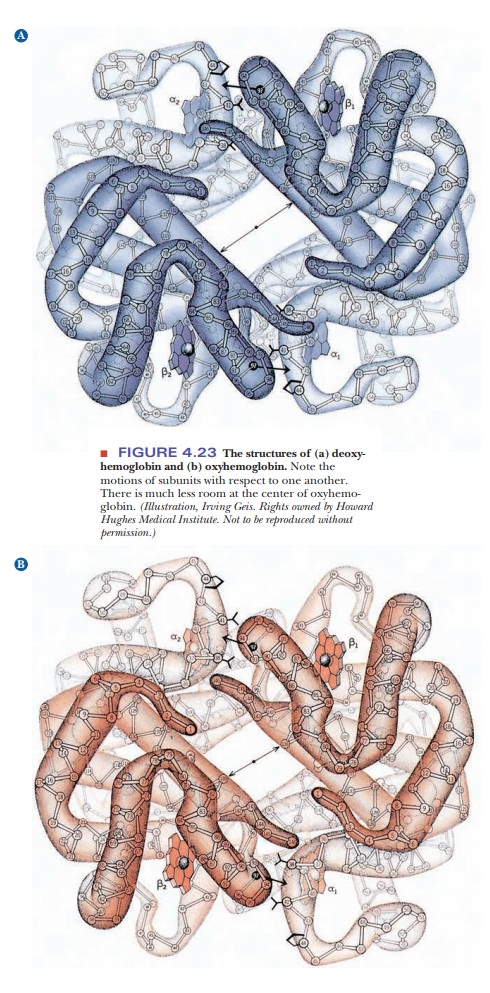
Structural
changes during binding of small molecules are characteristic of allosteric
proteins such as hemoglobin. Hemoglobin has different quaternary structures in
the bound (oxygenated) and unbound (deoxygenated) forms. The two β-chains are
much closer to each other in oxygenated hemoglobin than in deoxygenated
hemoglobin. The change is so marked that the two forms of hemoglobin have
different crystal structures (Figure 4.23).
Conformational Changes That Accompany Hemoglobin Function
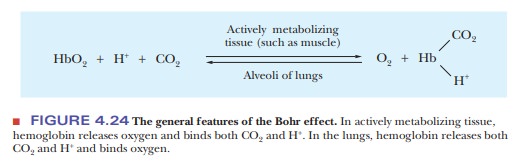
Other ligands are involved in cooperative
effects when oxygen binds to hemoglobin. Both H+ and CO2,
which themselves bind to hemoglobin, affect the affinity of hemoglobin for
oxygen by altering the protein’s three-dimensional structure in subtle but important
ways. The effect of H+ (Figure 4.24) is called the Bohr effect, after its discoverer,
Christian Bohr (the father of physicist Niels Bohr). The oxygen-binding ability
of myoglobin is not affected by the presence of H+ or of CO2.
An increase in the concentration of H+
(i.e., a lowering of the pH) reduces the oxygen affinity of hemoglobin.
Increasing H+ causes the protonation of key amino acids, including
the N-terminals of the α-chains and His146 of the β-chains. The
protonated histidine is attracted to, and stabilized by, a salt bridge to Asp94.
This favors the deoxygenated form of hemoglobin. Actively metaboliz-ing tissue,
which requires oxygen, releases H+, thus acidifying its local
environ-ment. Hemoglobin has a lower affinity for oxygen under these
conditions, and it releases oxygen where it is needed (Figure 4.25).
Hemoglobin’s acid–base properties affect, and are affected by, its
oxygen-binding properties. The oxygenated form of hemoglobin is a stronger acid
(has a lower pKa) than the
deoxygenated form. In other words, deoxygenated hemoglobin has a higher
affinity for H+ than does the oxygenated form. Thus, changes in the
quaternary structure of hemoglobin can modulate the buffering of blood through
the hemoglobin molecule itself.
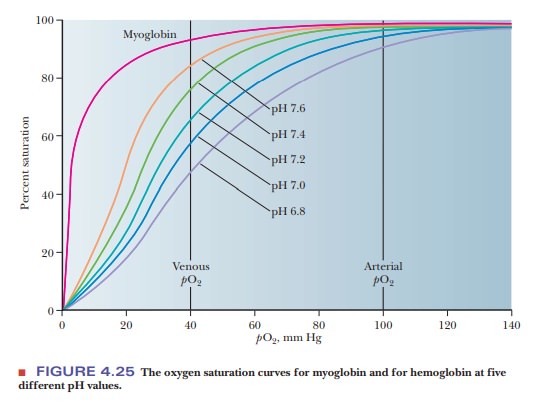
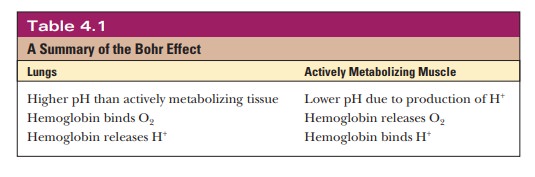
Table 4.1 summarizes the important features of
the Bohr effect.
Large amounts of CO2 are produced by
metabolism. The CO2, in turn, forms carbonic acid, H2CO3.
The pKa of H2CO3
is 6.35; the normal pH of blood is 7.4. As a result, about 90% of dissolved CO2
will be present as the bicarbonate ion, HCO3–, releasing
H+. (The Henderson–Hasselbalch equation can be used to confirm this
point.) The in vivo buffer system involving H2CO3 and HCO3–
in blood. The presence of larger amounts of H+ as a result of CO2
production favors the quaternary structure that is characteristic of
deoxygenated hemoglobin. Hence, the affinity of hemoglobin for oxygen is
lowered. The HCO3– is transported to the lungs, where it
combines with H+ released when hemoglobin is oxygenated, producing H2CO3.
In turn, H2CO3 liberates CO2, which is then
exhaled. Hemoglobin also transports some CO2 directly. When the CO2
concentration is high, it combines with the free α-amino groups to form
carbamate:
R-NH2 + CO2 < -- > R-NH-COO– + H+
This reaction turns the α-amino terminals into anions, which can then interact with the α-chain Arg141, also stabilizing the deoxygenated form.
In the presence of large amounts of H+
and CO2, as in respiring tissue, hemoglobin releases oxygen. The
presence of large amounts of oxygen in the lungs reverses the process, causing
hemoglobin to bind O2. The oxygenated hemoglobin can then transport
oxygen to the tissues. The process is complex, but it allows for fine-tuning of
pH as well as levels of CO2 and O2.
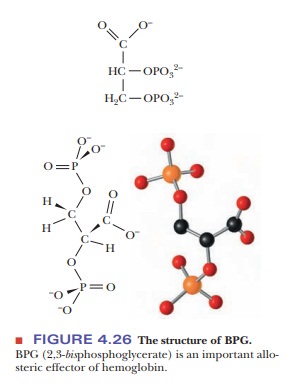
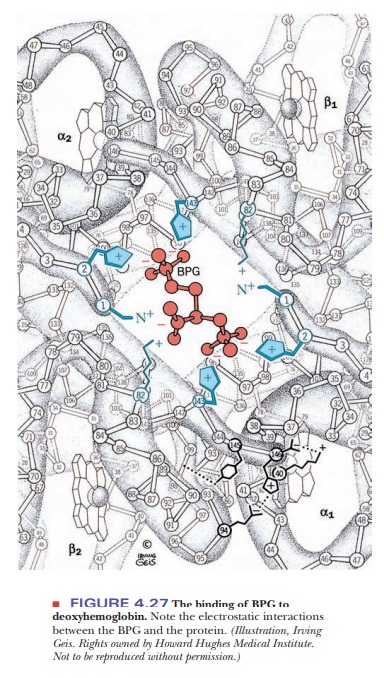
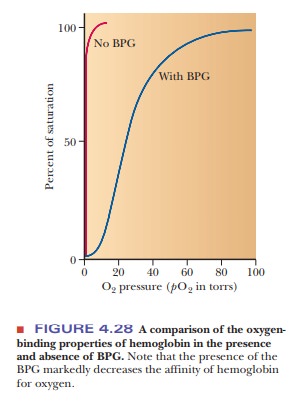
Hemoglobin in blood is also bound to another ligand, 2,3-bisphosphoglycerate(BPG) (Figure 4.26), with drastic effects on its oxygen-binding capacity. Thebinding of BPG to hemoglobin is electrostatic; specific interactions take place between the negative charges on BPG and the positive charges on the protein (Figure 4.27).
In the presence of BPG, the partial pressure at which 50% of hemoglobin is bound to oxygen is 26 torr.
If BPG were not present in blood, the oxygen-binding capacity of hemoglobin would
be much higher (50% of hemoglobin bound to oxygen at about 1 torr), and little
oxygen would be released in the capillaries. “Stripped” hemoglobin, which is
isolated from blood and from which the endogenous BPG has been removed,
displays this behavior (Figure 4.28).
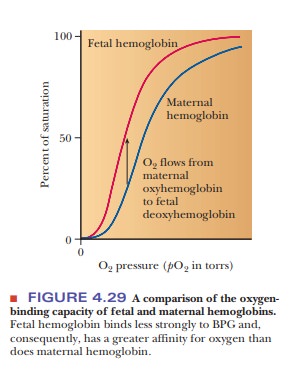
BPG also plays a role in supplying a growing
fetus with oxygen. The fetus obtains oxygen from the mother’s bloodstream via
the placenta. Fetal hemo-globin (Hb F) has a higher affinity for oxygen than
does maternal hemoglobin, allowing for efficient transfer of oxygen from the
mother to the fetus (Figure 4.29). Two features of fetal hemoglobin contribute
to this higher oxygen-binding capacity. One is the presence of two different
polypeptide chains. The subunit structure of Hb F is a2g2,
where the β-chains of adult hemoglobin (Hb A), the usual hemoglobin, have been
replaced by the γ-chains, which are simi-lar but not identical in structure.
The second feature is that Hb F binds less strongly to BPG than does Hb A. In
the β-chain of adult hemoglobin, His143 makes a salt bridge to BPG.
In the fetal hemoglobin, the γ-chain has an amino acid substitution of a serine
for His143. This change of a positively charged amino acid for a
neutral one diminishes the number of contacts between the hemoglobin and the
BPG, effectively reducing the allosteric effect enough to give fetal hemoglobin
a higher binding curve than adult hemoglobin.
Another type of hemoglobin that has been
studied extensively is sickle-cell hemoglobin, Hb S. In Hb S, the β-chains have a single amino acid substitution of a glutamic acid
for a valine. This substitution of a nonpolar amino acid for a polar one causes
the characteristic effects of the disease. The nonpolar amino acid is on the
surface and leads to aggregation of the molecules through non-polar
interactions. These aggregations lead to the sickling of the blood cells.
Summary
Quaternary structure is the
final level of protein structure and pertains to those proteins that consist of
multiple polypeptide chains. Each chain is called a subunit.
Subunits interact with each other through
non-covalent interactions.
Some proteins with multiple
subunits are allosteric, which means that the subunits interact such that
binding of a ligand to one subunit affects the binding of ligands to other
subunits.
Hemoglobin is a classic
example of protein quaternary structure. The protein has 4 subunits, two α-chains and two β-chains, and it exhibits
positive cooperativity. Binding of oxygen to one subunit makes it easier for
oxygen to bind to other subunits.
Hemoglobin’s affinity for
oxygen is controlled by several factors including oxygen pressure and pH. When
the pH drops or when oxygen pressure is low, hemoglobin tends to release more
oxygen to the tissues. When the pH is high and oxygen is plentiful, such as at
the lung-blood interface, hemoglobin binds oxygen.
Hemoglobin
is bound to 2,3-bisphosphoglycerate,
which acts as a bridge between the 4 subunits. In the absence of 2,3-bisphosphoglycerate, hemo-globin is not
allosteric and behaves like myoglobin.
Related Topics