Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Compounds of Phosphorus
Compounds of Phosphorus
a) Halides of Phosphorus
Phosphorus combines with allthehalogens forming phosphorus
halides which are all covalent compounds. Phosphorus chlorides are more
important. Tri and pentachlorides of
phosphorus are most common.
I. Phosphorus Trichloride, PCl3
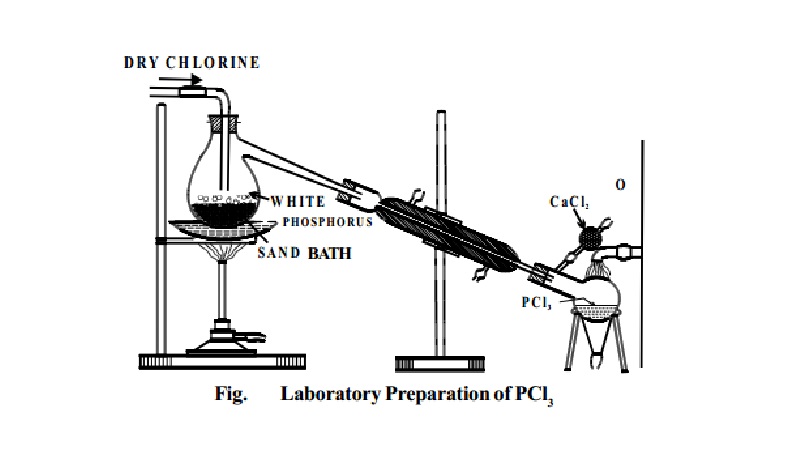
Preparation:
PCl3 is prepared by heating white phosphorus in a
current of dry chlorine.
P4 + 6Cl2 ® 4PCl3
Dry white phosphorus is placed in the retort and gently
heated on a water bath. A current of pure,
dry chlorine is led over the phosphorus. The phosphorus trichloride formed being volatile distils over and is
collected in a water cooled receiver.
The phosphorus trichloride obtained as above contains
some PCl5 as impurity. This is removed by distilling the PCl3 over white phosphorus.
Physical
properties
1. Colourless
low boiling liquid
2. It fumes
in moist air
3. It has
pungent odour.
Chemical
Properties
1. It is violently hydrolysed by water giving phosphorus
acid and hydrochloric
acid gas.
PCl3 + 3 H2O ® 3HCl + H3PO3
In a similar manner it reacts with organic compounds
containing hydroxyl (OH)
group, such as acids and alcohols.
PCl3 + 3CH3COOH ® 3CH3COCl + H3PO3
Acetic Acid Acetyl
Chloride
PCl3 + 3C2H5OH ® 3 C2H5Cl
+ H3PO3
Ethyl alcohol Ethyl
Chloride
2.. It reacts with
chlorine or sulphuryl chloride forming phosphorus pentachloride.
PCl3 + Cl2 ® PCl5
PCl3 + SO2Cl2 ® PCl5 + SO2
3. It readily combines with oxygen forming phosphorus
oxychloride
2PCl3 + O2 ®2POCl3
4. It reacts with SO3 to form phosphorus oxychloride and SO2
SO3 + PCl3 ® POCl3 + SO2
Structure:
PCl3 molecule has a pyramidal shape, which arises
from sp3
hybridisation of phosphorus atom. One of the tetrahedral
positions is occupied by a lone pair of
electrons.

II. Phosphorus pentachloride, PCl5
Preparation:
Phosphorus pentachloride is usually prepared by the action of an
excess of chlorine on phosphorus trichloride.
PCl3 + Cl2 ® PCl 5
Physical properties
1.
Phosphorus
pentachloride is a yellowish white crystalline solid.
2.
It sublimes on
heating at 473 K and melts at 318 K under pressure.
Chemical properties
Phosphorus pentachloride dissociates on heating into
phosphorus trichloride and chlorine.
PCl5 -- > < --- PCl3 + Cl2
It is violently hydrolysed by water giving phosphorus
oxychloride or
phosphoric acid depending upon the quantity of water.
PCl5 + H2O -- insufficient water --- > POCl3 + 2HCl
PCl5 + 4H2O -- Excess of water -- > H3PO4 + 5HCl
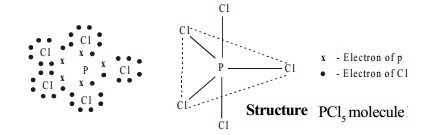
Structure
PCl5 molecule has trigonal bipyramidal shape in vapour state which arises from sp3d hybridisation of phosphorus atom.
b) Oxides
of phosphorus
I Phosphorus
trioxide P2O3 or P4O6
It is obtained by the combustion of phosphorus in a
limited supply of air.
4P + 3O2 ® 2P2O3
Physical properties
1. It is a white waxy substance
2. It
has a garlic odour.
Chemical properties
1. It reacts with cold water, gives phosphorus acid.
P2O3 +3H2O ® 2H3PO3
2. It reacts with hot water vigorously to form
inflammable phosphine.
2P2O3 + 6H2O ® PH3 + 3H3 PO4
II Phosphorus pentoxide P2O5 or P4O10
Phosphorus pentoxide can be prepared by burning
phosphorus with sufficient
supply of air.
P4 + 5O2 ®P4 O10
Physical properties
It is a white solid and an acidic oxide.
Chemical properties
1. It reacts with moisture to form metaphosphoric
acid.
P4O10 + 2H2O ® 4HPO3
When
the solution is boiled, the metaphosphoric acid is changed to
orthophosphoric acid.
HPO3 + H2O ® H3PO4
or
P4O10 + 6H2O ® 4H3 PO4
2.
Phosphorus pentoxide extracts water from many inorganic compound
including sulphuric acid, nitric acid and several
organic compounds. It is therefore, used
as a powerful dehydrating agent.
Use: It is used
as a dehydrating agent.
c) Oxy-Acids of Phosphorus
I. Phosphorus acid - H3PO3
It is prepared by the action of cold water on phosphorus
(III) oxide or phosphorus (III)
chloride.
P2O3 + 3H2O ® 2H3PO3
PCl3 + 3H2O ® H3PO3 + 3HCl
Physical properties
It is a white crystalline solid with garlic taste.
Chemical Properties
1. Acidic nature: It is a dibasic acid and gives salts of two types.
H3PO3 + NaOH ® NaH2PO3 + H2O
Sodium dihydrogen Phosphite
H3PO3 + 2NaOH ®
Na2HPO3 + 2H2O
Disodium hydrogen Phosphite
2.
When it is heated it undergoes auto-oxidation and reduction to form
phosphoric acid and phosphine.
D
4H3 PO3 ® 3H3PO4 + PH3
3. It is a powerful reducing agent because it has P-H
bond. It reduces silver
nitrate solution into silver.
2AgNO3 + H3PO3 + H2O ® 2Ag +H3PO4+2HNO3
Electronic structure
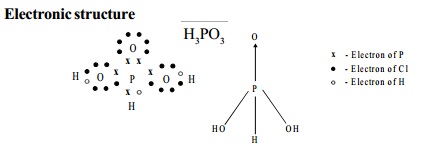
Use: It is used
as a reducing agent
II. Ortho phosphoric Acid, H3PO4
Preparation
1. It is prepared by dissolving phosphorus pentoxide in
water and boiling the
solution.
P2O5 + 3H2O ® 2H3PO4
2. Laboratory preparation: In the laboratory
orthophosphoric acid can be
prepared by boiling a mixture of red phosphorus with 50%
nitric acid in a flask fitted with a
reflux condenser on a water bath till no more oxides of nitrogen are liberated.
Iodine acts as a catalyst. The product is evaporated
below 453 K and then
cooled in a vaccum desiccator surrounded by freezing
mixture when crystals of orthophosphoric
acid are deposited.
P+5HNO3 ® H3PO4 +5NO2 +H2O
Physical properties
1. It is a deliquescent crystalline solid.
2. It is soluble in water.
Chemical properties
1. It is a tribasic acid. It combines with alkalies like
NaOH to form three series
of salts.
H3PO4 +NaOH ® NaH2PO4 + H2O
Sodium Di hydrogen Phosphate
H3PO4 +2NaOH ® Na2HPO4 + 2H2O
Disodium hydrogen Phosphate
H3PO4 + 3NaOH ® Na3PO4 + 3H2O
Sodium Phosphate
2.
On heating it gives pyrophosphoric acid at 523 K and at 589 K gives
metaphosphoric acid
523K 589K
H3PO4 H4 P2O7 2HPO3 + H2O
3. On reaction with silver nitrate, it gives yellow
precipitate of silver phosphate.
H3PO4 + 3AgNO3 ® Ag3PO4+3HNO3
Uses
1. It is used in the preparation of HBr and HI as a
substitute for sulphuric acid.
2. It is used as souring agent in the preparation of soft
drinks.
3. It is used in the preparation of phosphate salts of
sodium, potassium and ammonium.
4.
It is used in the
manufacture of phosphatic fertilisers.
Structure
Being a tribasic acid, the structure of phosphoric acid
is represented as
III. B. Pyrophosphoric acid, H4 P2 O7
Preparation:
Pyrophosphoric acid is prepared by heating orthophosphoric
acid to 523 K - 533 K.
2H3PO4 ® H4P2O7 + H2O
Physical Properties
It is a colourless crystalline solid.
Chemical Properties
1. It is reconverted to orthophosphoric acid on
boiling with water
H4P2O7 + H2O ® 2H3PO4
2. When heated strongly, it yields metaphosphoric
acid
H4P2O7 2HPO3 + H2O
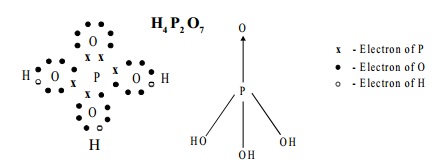
Structure:
The Structure of pyrophosphoric acid is represented as:
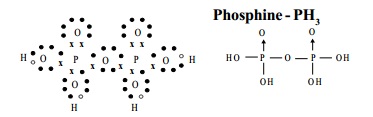
d) Phosphine - PH3
Phosphine is the best known hydride of phosphorus.
Laboratory preparation: It is usually obtained by boiling white phosphorus with 30-40% solution of caustic soda in an inert
atmosphere of CO2.
4P + 3NaOH + 3H2O ® PH3 + 3NaH2PO2
Sodium hypophosphite
Phosphine so obtained is impure. It is passed into an
aqueous solution of hydrogen iodide, PH4I is formed.
PH4I is
heated with KOH or NaOH, pure
phosphine is obtained.
PH3 + HI ® PH4I
PH4I + NaOH ® PH3 + NaI + H2O
Physical properties
Phosphine is colourless gas with rotten fish odour.
Chemical properties
1. Dissociation:
Phosphine dissociates at about 723 K and gives red
phosphorus.
2. Action of air: It burns with oxygen and produces phosphorus pentoxide.
3. Action of chlorine: Phosphine burns in chlorine spontaneously forming
PCl3 and PCl5.
PH3 + 3Cl2 ® PCl3 + 3HCl PH3 + 4Cl2 ® PCl5 + 3HCl
4. Reducing properties: PH3 is a powerful reducing
agent. When it is passed
through the salt solutions, corresponding metal is
formed.
PH3 + 6AgNO3+ 3H2O ® 6Ag + 6HNO3 + H3PO3
Uses
1. Smoke screens
When PH3 burns it
produces smoke which is dense enough to serve as
smoke screens.
2. Holme's signal : Containers which have a
perforated bottom and a hole at
the top are filled with calcium phosphide and calcium
carbide. These are thrown into the sea.
Water enters the container through the bottom and reacts with calcium carbide and calcium phosphide to give acetylene and
phosphine. Phosphine gets ignited
spontaneously as it comes in contact with air and also ignites acetylene. Thus a bright red flame is produced which is accompanied
by huge smoke due to the burning of
phosphine. This serves as a signal to the approaching ships.
Ca3P2 + 6H2O ® 2 PH3 +
3Ca(OH)2
CaC2 + 2H2O ® C2H2 + Ca(OH)2
Problem
An element 'A' occupies group number 15 and period
number 3 reacts
with chlorine to give B which further reacts with
chlorine to give C at 273 K. Both
B and C are chlorinating agent for organic compounds. C is a better
chlorinating agent because it chlorinates metals also.
B reacts with SO3 and
reduces it to SO2. B has a pyramidal shape. C
has trigonal bipyramidal shape by
sp3d hybridisation. Identify the element A and the compounds B and
C. Write
the reactions.
1. The element which occupies group number 15 and
period number 3 is
phosphorus. Therefore A is phosphorus. Phosphorus reacts
with chlorine
to give PCl3. Therefore compound B is phosphorus trichloride
and it has a
pyramidal shape.
P4 + 6Cl2 ® 4PCl3
2. PCl3 further
reacts with Cl2 to give PCl5. Therefore, the compound C is
phosphorus pentachloride and it has a trigonal
bipyramidal shape.
PCl3 + Cl2 ® PCl5
3. PCl3 and PCl5 are chlorinating agents for organic compounds.
So, both
reacts with C2H5OH gives C2H5Cl.
PCl3 + 3C2H5OH ® 3C2H5Cl+ H3PO3
PCl5 + C2H5OH ® C2H5Cl + POCl3 + HCl
4. PCl5 is a
better chlorinating agent. So it chlorinates copper.
PCl5 + 2Cu ® 2CuCl + PCl3
5. PCl3 reacts
with SO3 and reduces it to SO2.
PCl3 + SO3 ® POCl3 + SO2
Related Topics