Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Halides of Phosphorus: Phosphorus TrichlorideII. Phosphorus pentachloride - Preparation, properties, Structure
Phosphorus -
Atomic Number : 15
Electronic Configuration : [Ne]
3s2 3p3 Group Number : 15 Periodic Number : 3
Halides of Phosphorus (Compounds of Phosphorus)
Phosphorus combines with allthehalogens forming phosphorus
halides which are all covalent compounds. Phosphorus chlorides are more
important. Tri and pentachlorides of
phosphorus are most common.
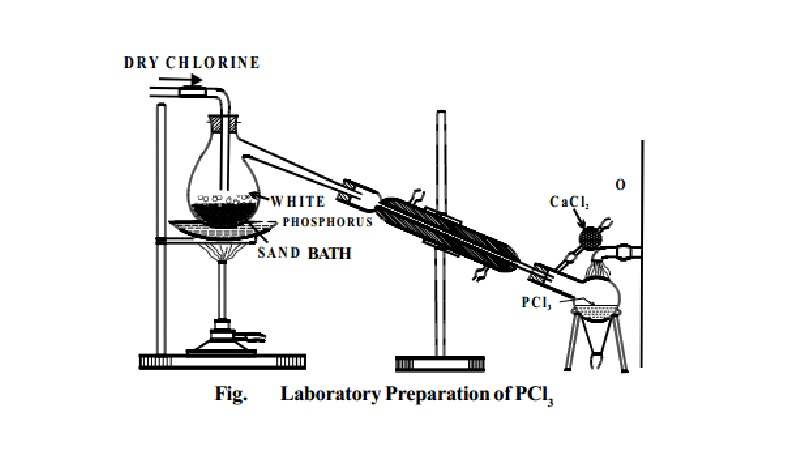
I. Phosphorus Trichloride, PCl3
Preparation:
PCl3 is prepared by heating white phosphorus in a
current of dry chlorine.
P4 + 6Cl2 ® 4PCl3
Dry white phosphorus is placed in the retort and gently
heated on a water bath. A current of pure,
dry chlorine is led over the phosphorus. The phosphorus trichloride formed being volatile distils over and is
collected in a water cooled receiver.
The phosphorus trichloride obtained as above contains
some PCl5 as impurity. This is removed by distilling the PCl3 over white phosphorus.
Physical
properties
1. Colourless
low boiling liquid
2. It fumes
in moist air
3. It has
pungent odour.
Chemical
Properties
1. It is violently hydrolysed by water giving phosphorus
acid and hydrochloric
acid gas.
PCl3 + 3 H2O ® 3HCl + H3PO3
In a similar manner it reacts with organic compounds
containing hydroxyl (OH)
group, such as acids and alcohols.
PCl3 + 3CH3COOH ® 3CH3COCl + H3PO3
Acetic Acid Acetyl
Chloride
PCl3 + 3C2H5OH ® 3 C2H5Cl
+ H3PO3
Ethyl alcohol Ethyl
Chloride
2.. It reacts with
chlorine or sulphuryl chloride forming phosphorus pentachloride.
PCl3 + Cl2 ® PCl5
PCl3 + SO2Cl2 ® PCl5 + SO2
3. It readily combines with oxygen forming phosphorus
oxychloride
2PCl3 + O2 ®2POCl3
4. It reacts with SO3 to form phosphorus oxychloride and SO2
SO3 + PCl3 ® POCl3 + SO2
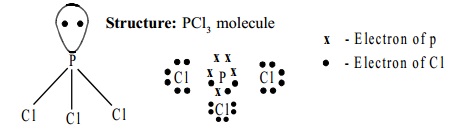
Structure:
PCl3 molecule has a pyramidal shape, which arises
from sp3
hybridisation of phosphorus atom. One of the tetrahedral
positions is occupied by a lone pair of
electrons.
II. Phosphorus pentachloride, PCl5
Preparation:
Phosphorus pentachloride is usually prepared by the action of an
excess of chlorine on phosphorus trichloride.
PCl3 + Cl2 ® PCl 5
Physical properties
1.
Phosphorus
pentachloride is a yellowish white crystalline solid.
2.
It sublimes on
heating at 473 K and melts at 318 K under pressure.
Chemical properties
Phosphorus pentachloride dissociates on heating into
phosphorus trichloride and chlorine.
PCl5 -- > < --- PCl3 + Cl2
It is violently hydrolysed by water giving phosphorus oxychloride or phosphoric acid depending upon the quantity of water.
PCl5 + H2O -- insufficient water --- > POCl3 + 2HCl
PCl5 + 4H2O -- Excess of water -- > H3PO4 + 5HCl
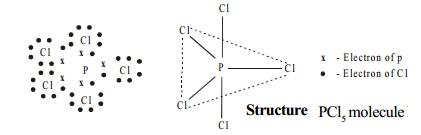
Structure
PCl5 molecule has trigonal bipyramidal shape in vapour state which arises from sp3d hybridisation of phosphorus atom.
Related Topics