Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Group - 15 Elements - The Nitrogen Family
GROUP - 15 ELEMENTS - THE NITROGEN FAMILY
The group 15 (VA) elements are nitrogen, phosphorus, arsenic, antimony and bismuth.
1.
Nitrogen is a gas.
It makes up 78% of the earth's atmosphere by volume.
2.
Phosphorus is the
most abundant element of 15th group,
accounting for 0.10% of the mass
of the earth's crust.
3.
Arsenic is also
used to make pesticides and semi conductors, such as GeAs.
4.
Bismuth is a silvery solid. Bismuth
compounds are present in some pharmaceuticals
such as Pepto-bismol.
5.
The natural
abundance of As, Sb and Bi in the earth's crust is relatively low.
General trends
Electronic configuration: All these elements have general electronic configuration
of ns2 np3.
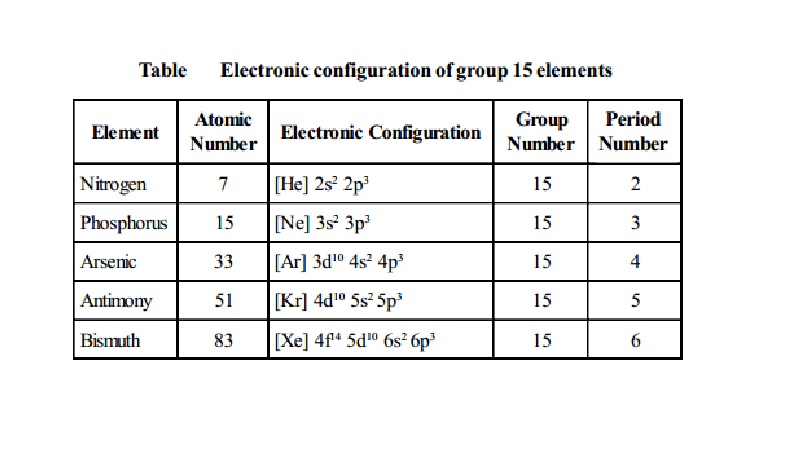
Electronic configuration of group 15 elements
Nitrogen -
Atomic Number : 7
Electronic Configuration : [He]
2s2 2p3 Group Number : 15 Periodic Number : 2
Phosphorus -
Atomic Number : 15
Electronic Configuration : [Ne]
3s2 3p3 Group Number : 15 Periodic Number : 3
Arsenic -
Atomic Number : 33
Electronic Configuration : [Ar]
3d10 4s2 4p3 Group Number : 15 Periodic Number : 4
Antimony -
Atomic Number : 51
Electronic Configuration : [Kr]
4d10 5s2 5p3 Group Number : 15 Periodic Number : 5
Bismuth -
Atomic Number : 83
Electronic Configuration : [Xe] 4f14 5d10 6s2 6p3 Group Number : 15 Periodic Number : 6
Compounds of Phosphorus
a) Halides of Phosphorus
Phosphorus combines with allthehalogens forming phosphorus halides which are all covalent compounds. Phosphorus chlorides are more important. Tri and pentachlorides of phosphorus are most common.
b) Oxides
of phosphorus
Phosphorus
trioxide P2O3 or P4O6
It is obtained by the combustion of phosphorus in a
limited supply of air.
4P + 3O2 ® 2P2O3
c) Oxy-Acids of Phosphorus
Phosphorus acid - H3PO3
It is prepared by the action of cold water on phosphorus (III) oxide or phosphorus (III) chloride.
d) Phosphine - PH3
Phosphine is the best known hydride of phosphorus.
Related Topics