Chapter: The Massage Connection ANATOMY AND PHYSIOLOGY : Introduction to Anatomy and Physiology
Chemical Bonds
Chemical Bonds
Atoms can interact in three ways; therefore, there are three types of chemical bonds—ionic bonds, covalent bonds, and hydrogen bonds.
Ionic Bonds
Some atoms may lose or gain an electron when bond-ing with another atom. In the first case, this atom has one electron less than the number of protons, mean-ing that there are more positive than negative charges. This atom is referred to as a cation (posi-tively charged). In the second case, this atom gains an electron and has more electrons than protons, meaning that there are more negative charges than positive charges. This atom is referred to as an an-ion. Both cations and anions, with their unequalnumber of protons and electrons, are known as ions. Ions are denoted by their chemical symbol, with pos-itive or negative signs given in superscript. For ex-ample, sodium ion is represented as Na ; chlorine as Cl-. Being positively charged, cations attract anions and vice versa. This type of bonding is known as ionic bonds. Ionic bonds and ions are especially im-portant in nerve conduction and brain activity.
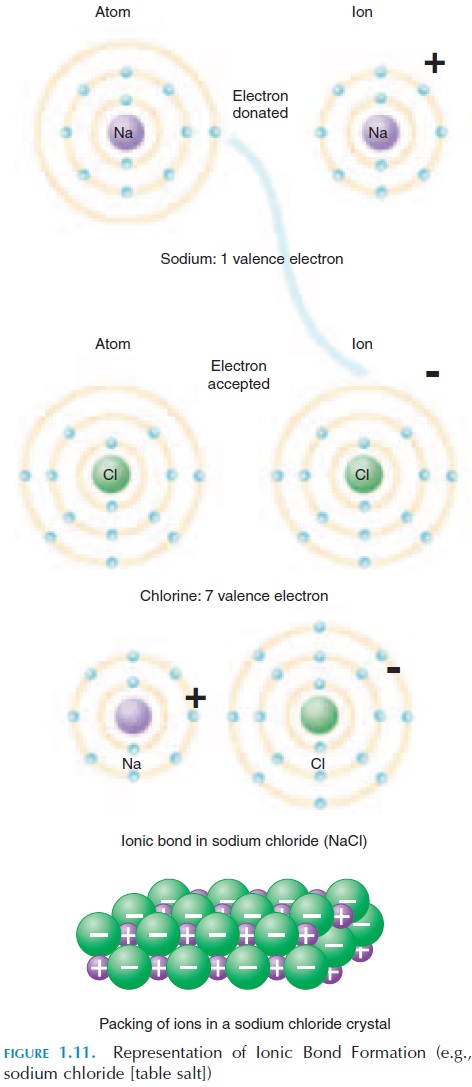
The formation of salt—table salt is a good example (see Figure 1.11). The chemical name of table salt is sodium chloride (NaCl); it is made up of sodium and chlorine. Sodium has an atomic number of 11. This means that it has 11 protons (and 11 electrons) to be neutral. Of the eleven electrons, two occupy the first energy level and eight occupy the second energy level and fill it. The remaining one electron (2 8 10) or-bits alone in the third energy level. Because the outer energy level is not full, the sodium atom is reactive and tends to donate its electron to another atom.
Chlorine has an atomic number of 17. This means it has 17 protons and 17 electrons, making it neutral. Of the electrons, two occupy the first energy level, eight occupy the second level, and seven occupy the outer level. One more electron will fill its outer energy level; chlorine has a tendency to attract an electron.
By ionic bond, sodium and chlorine come together to satisfy each other’s needs. Sharing electrons, the positively charged sodium is attracted to the nega-tively charged chlorine and they stay together to form the compound sodium chloride—table salt. When sodium chloride is dissolved in water, the ions sepa-rate—ionize—and positively charged sodium ions (Na ) and negatively charged chloride ions (Cl-) are found.
Covalent Bonds
In some cases, atoms share their electrons rather than gain or lose them. Such bonds are known as co-valent bonds (see Figure 1.12). The resultant chemi-cal is termed amolecule. A good example is the ele-ment hydrogen. Hydrogen has an atomic number of 1 (i.e., 1 proton and 1 electron). However, the outer
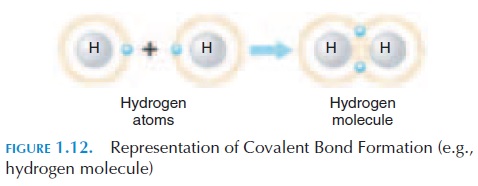
shell needs another electron to be complete. There-fore, one hydrogen atom shares its electron with an-other hydrogen atom, completing their energy levels and forming a molecule. This is how hydrogen nor-mally exists—in pairs, and is referred to as hydrogen molecules. A hydrogen molecule is symbolized as H2.
Similarly, many different elements may bond to-gether. Carbon dioxide gas has one carbon atom and two oxygen atoms bonded covalently (CO2). Water has two hydrogen atoms and one oxygen atom bonded covalently (H2O). Elements may share one, two, or three electrons. In the human body, covalent bonds are the most common.
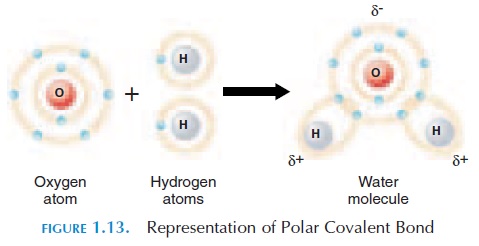
When covalent bonds are formed, the electrons may be shared equally or unequally between the specific atoms. When shared equally, these bonds are known as nonpolar covalent bonds. Sometimes, one atom at-tracts the shared electron more than the other atom. In this case, the atom that attracts the electron to a greater degree would be slightly more negatively charged than the other atom. The other atom will be slightly more positively charged. These charges are represented with the symbol or - (see Figure 1.13). These covalent bonds are known as polar covalent bonds.
Hydrogen Bonds
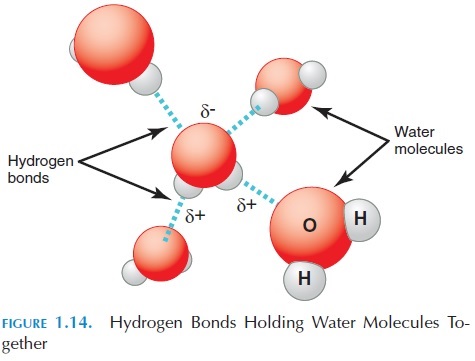
Other than ionic and covalent bonds, other weak at-tractions may be present between atoms of the same molecule or compound or between atoms in other molecules. The most important of these weak attrac-tions are hydrogen bonds, in which a hydrogen atom involved in a polar covalent bond is attracted to oxy-gen or nitrogen involved in a polar covalent bond by itself. This attraction is important. Although mole-cules are not formed through the hydrogen bonds, this bonding can alter the shape of the molecules. For example, it is this weak attraction that holds water together and makes it form a drop. We refer to this force as surface tension (see Figure 1.14). At the molecular level in the body, these hydrogen bonds can alter the properties of proteins, making them change their shape and structure.
Matter exists as solids, liquids, or gases as a result of the degree of interaction between the atoms and molecules. For example, hydrogen molecules do not attract each other and, therefore, exist as gas. Water, however, has more interactions and exists as liquid throughout a wide temperature range.
Related Topics