Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Characteristics of entropy 'S'
Characteristics of entropy 'S'
i.
The term 'S' entropy is evolved from the
formulation of II law of thermodynamics as a
thermodynamic state function.
ii.
Entropy change 'DS' of a system under a process is defined as the constant
equal to the ratio of the heat change accompanying a
process at constant temperature to the temperature of the system under process.
The process should be reversible at that
temperature.
DSrev = Dq rev / T(K)
Heat, q is not a state function , But for a reversible
process Dq = (q2-q1) divided by temperature (T) of the process is a state
function.
iii.
A spontaneous
process is accompanied by increase in the 'disorder' (or) 'randomness' of the molecules constituting the system.
Entropy increases in all spontaneous processes.
Hence entropy may be regarded as a measure of disorder (or) randomness of the molecules of the system.
iv.
When a system
undergoes a physical (or) a chemical process, there occurs a change in the entropy of the system and also in its
surroundings. This total change in the
entropy of the system and its surroundings is termed as the entropy change of the universe brought about by the process. For
an isothermal process (T=constant), the
entropy change of the universe during a reversible process is Zero.
The entropy of the universe increases in an irreversible
process.
v.
The energy of the
universe remains constant although the entropy of the universe tends to a maximum.
vi.
For a spontaneous
process, at constant T, DS is positive (DS > 0).
DS is positive (DS > 0). For an equilibrium process, DS is zero.
For a non spontaneous process,
DS is negative or (DS < 0).
vii)
Units of entropy: The dimension of entropy are energy in terms of heat X temperature-1. The entropy is expressed as calories
per degree which is referred to as
the entropy units (eu). Since entropy also depends on the quantity of the substance, unit of entropy is calories per
degree per mole (or) eu. per mole. cgs units of entropy is cal.K-1 denoted as eu. The SI unit is JK-1 and denoted EU. 1 eu = 4.184 EU.
viii) Entropy change is related to enthalpy change as
follows:
For a reversible and isothermal process,
DSrev = Dq rev / T
Since DH is the heat absorbed (or) evolved in the process at constant T and pressure P.DS is also calculated from DH as DS = DH / T where T is the temperature
of the process involving DH, amount of enthalpy change, at constant pressure.
Standard Entropy
The absolute entropy of a pure substance at 25 o C (298 K)
and 1 atm pressure is called the
standard entropy, S o . The standard entropies of all substances either elements
or compounds at any temperature above 0 o K always have positive values.
When the standard entropies, S o of various substances
are known, the standard entropy
change of a chemical process or reaction is written as
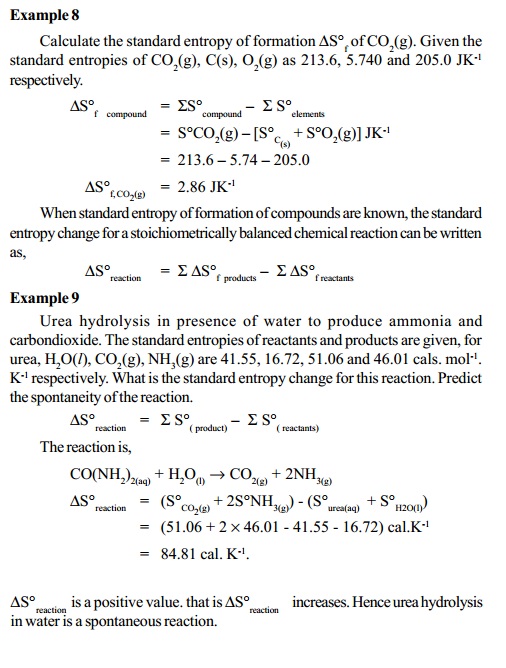
DS o = S S o products - S S o reactants
This DS o is the standard entropy change of the reaction.
Standard entropy change of formation, DS o f is defined as the entropy of formation
of 1 mole of compound from the elements present in the standard conditions. DS o fcan be
calculated for chemical compounds using the S o values of elements from which the compound is formed. DS o f compound = SS o compound - S S o elements
Related Topics