Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Gibbs free energy 'G' and Standard free energy (G°)
Gibbs free energy 'G'
According to II law of thermodynamics, inorder to predict the spontaneity of a process entropy of universe is considered. DSuniverse is the sum of DSsystemand DSsurroundings. It is difficult to determine DSsurroundings in most of the physical and chemical processes. Therefore a thermodynamic function which reformulates the spontaneity criterion considering only the system under study is required.
For this purpose, "a free energy function" has
been introduced by II law of thermodynamics.
The free energy function, called the Gibbs free energy function, denoted by the symbol 'G' is mathematically defined as,
G = H - TS
where H = enthalpy or heat content of the system, T =
Temperature in Kelvin and S = entropy
This expression is valid for constant temperature and
pressure processes.
In an isothermal process, if DH and DS are the changes in enthalpy and entropy of the system, then free energy change DG is given by,
DG = DH - TDS
If 1 and 2 refer to the initial and final states of the
system during the isothermal process, then
DG = (G2-G1) = (H2-H1) - T(S2-S1)
from I law of thermodynamics
DH = DE + PDV
Therefore DG = DE + PDV - TDS.
For a spontaneous process, the enthalpy change at
constant pressure will be negative. This
is because in an exothermic process, the enthalpy of the final state (H2) is lower than the enthalpy of the initial
state (H1)
so that (H2-H1) is negative and the process take place spontaneously to
attain the lower enthalpy state.
Similarly, the entropy change (DS) increases in a spontaneous process since entropy of the final state S2 will be greater than the initial state S1 so that (S2-S1) = DS, is positive. Combining negative DH and positive DS, in the expression
for free energy change DG, at constant temperature, the overall
magnitude of DG becomes negative
for a spontaneous process. Here, DH and DS terms refer only to the system.
DG = DH - TDS
Hence, criterion for the prediction of feasibility of a
reaction (or) the prediction of
thermodynamic spontaneity of a process based on the free energy change (DG)
of the process is given as : when at constant temperature and pressure of the
system, if,
DG
< 0, DG is -ve, the process is spontaneous and feasible
i.e. DG = 0, the process is in equilibrium
i.e. DG < 0, DG is +ve, the
process is non spontaneous and non feasible.
In chemical thermodynamics, spontaneous processes are also
known as
irreversible (or) feasible processes while non
spontaneous processes are known as non feasible processes since time factor of
the process is not considered here.
All reversible processes are considered as equilibrium
processes.
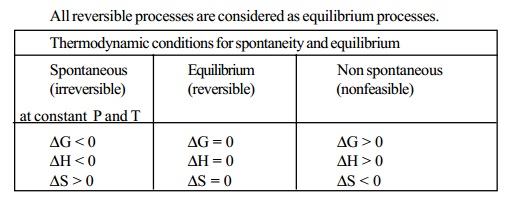
Thermodynamic
conditions for spontaneity and equilibrium
Spontaneous (irreversible)
at constant P and T
DG < 0
DH < 0
DS > 0
Equilibrium (reversible)
DG = 0
DH = 0
DS = 0
Non spontaneous (nonfeasible)
DG > 0
DH > 0
DS < 0
Characteristics of Free energy 'G'
i) G is defined as (H-TS) where H and S are the enthalpy
and entropy of the system
respectively. T = temperature. Since H and S are state functions, G is a state function.
i) G is an extensive property while DG = (G2-G1) which is
the free energy change between the
initial (1) and final (2) states of the system becomes the intensive property when mass remains constant between
initial and final states (or) when the
system is a closed system.
iii) G has a single value for the thermodynamic state
of the system.
iv) G and DG values correspond
to the system only. There are three cases of DG in predicting the nature of the process. When, DG<0 (negative), the process
is spontaneous and feasible; DG = 0. The process is in equilibrium and DG > 0 (positive), the process is nonspontaneous and
not feasible.
v) DG
= DH - TDS. But according to
I law of thermodynamics,
DH = DE + PDV and DE = q - w.
∴ DG = q - w + PDV
- TDS
But DS = q/T and TDS = q = heat involved in the process.
∴ DG = q - w + PDV
- q = -w + PDV
(or) -DG = w - PDV
= network.
The decrease in free energy -DG, accompanying a process taking place at constant
temperature and pressure is equal to the maximum obtainable work from the system other than work of expansion.
This quantity is called as the "net work" of
the system and it is equal to (w - PDV).
∴ Net work = -DG = w - PDV.
-DG
represents all others forms of work obtainable from the system such as
electrical, chemical or surface work etc other than P-V work.
Standard free energy (G o )
Like standard enthalpy of formation of substances,
standard enthalpy change of a reaction,
standard free energy of formation of substances and standard free energy change
of reactions are considered. The standard free energy value (G o ) of all
substances either elements or compounds may be calculated from H o and S o values at standard conditions of temperature (298 K)
and pressure (1 atm) and the substance
being present in the standard state.
i.e G o = H o - TS o
Standard free energies of formation of elements are taken
as zero. Hence, standard free
energy change of a reaction which is stoichiometrically balanced, is equal to the difference between the total sum of the
standard free energies of products and
the total sum of the standard free energies of reactants, at standard conditions.
DG o reaction = SG o product - SG o reactants
DG o reaction can also be
calculated from DH o reaction and DS o reaction values. DH o reaction and DS o reaction can be calculated
from H o f and S o values of respective product and reactant molecules at the constant temperature and pressure.
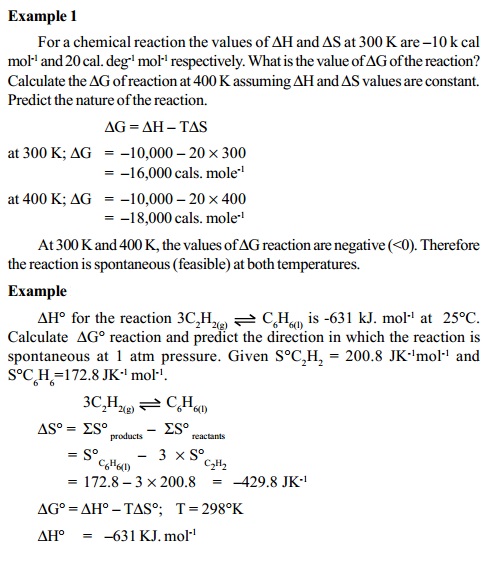
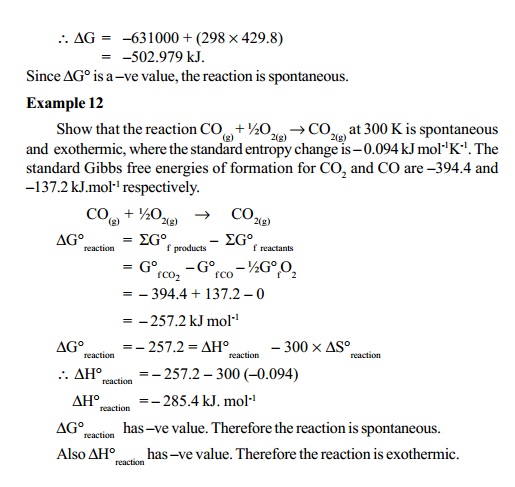
Related Topics