Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Entropy And Entropy Change
ENTROPY AND ENTROPY CHANGE
The entropy function 'S' introduced in the second law of
thermodynamics is explained as
below.
Entropy function 'S' represents the ratio of the heat
involved (q) to the temperature (T) of the process. That is S = q/T . This relation is valid only for reversible processes. If a system is changed from state 1
to state 2 at constant temperature and if dq rev is the heat involved in the process, then
entropy change of the process (DS) is given by,
S 2 - S1 = D S
= ò12 (dq rev / T)
where the process is a reversible one. Entropy (S) and
the change in entropy of the process (DS) are each state functions even though q and dq path functions.
In a reversible process, entropy of universe remains a
constant.
S universe = S system + S surroundings = Constant
Entropy change (DS) can be derived for various thermodynamic processes as below:
Isothermal process (T = constant)
DS = ò12 (dq rev / T) = (q2 - q1 ) / T
Isothermal and isobaric process (T and P = Constant)
ò12 (dq P, rev / T)
Isothermal, and isochoric process (T and V- Constant)
ò12 (dq V, rev / T)
The term 'natural process means that the process is
spontaneous and does not need to be
induced. In order to find out whether a process is spontaneous or not, the entropy changes of the system and the
surroundings for the stipulated process
is considered. If this change is positive i.e. if the entropy of the universe
increases, the process will take place spontaneously and irreversibly.
If the entropy change of the universe is zero or negative
(DS<0) the system will behave as non spontaneous.
When the external pressure is less than the internal
pressure of a gaseous system, the gas
expands spontaneously. When volume increases in expansion, the disorder in the movement of gaseous molecules
increases. Thus, spontaneous processes are
associated with increase in disorder. When disorder in a process is favoured it occurs spontaneously and we say, the
entropy change is positive. Entropy is a measure of microscopic disorder in the
system and also represents spontaneity.
For a reversible process,
DS system = - DS surroundings
∴DSuniverse= DS system + DS surroundings = 0.
For an irreversible
(spontaneous) process.
DSuniv> 0
(positive value)
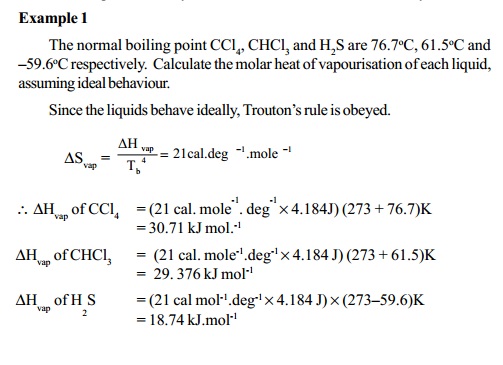
Entopy change in a physical
(phase) transformation can be determined particularly
for evaporation of liquids at the boiling points, using Trouton's rule.According to this rule, the heat of vaporisation (DHvap)in calories per mole divided by
the boiling point of the liquid in Kelvin is a constant equal to 21 cal deg-1 mole-1 and is
known as the entropy of vapourisaiton.
DSvap = 21cal.deg-1mole-1
DHvap = Enthalpy change of vapourisation = Latent
heat of vapourisation.
This equation is useful for estimating the molar heat of
vaporization of a liquid of known boiling
point. Substances that deviate from this rule are as follows:
i) Low boiling liquids such as hydrogen and Helium which
boil only a little above 0K.
ii) Polar substances like water, alcohol which form
hydrogen bonded liquids and exhibit very
high boiling points as well as high DHvap.
iii) Liquids such as acetic acid whose molecules are
partially associated in the vapor phase
and possess very low entropy vaporization which is very much less than 21 cals/ mol/deg. Those liquids that obey Troutons rule are said to behave
ideally.
Related Topics