Chapter: Biochemistry: Lipid Metabolism
Catabolism of Lipids
Catabolism of Lipids
The oxidation of fatty acids is the chief
source of energy in the catabolism of lipids; in fact, lipids that are sterols
(steroids that have a hydroxyl group as part of their structure;) are not
catabolized as a source of energy but are excreted. Both triacylglycerols,
which are the main storage form of the chemical energy of lipids, and
phosphoacylglycerols, which are important components of biological membranes,
have fatty acids as part of their covalently bonded structures. In both types
of compounds, the bond between the fatty acid and the rest of the molecule can
be hydrolyzed (Figure 21.1), with the reaction catalyzed by suitable groups of
enzymes—lipases, in the case of triacylglycerols,
and phospholipases, in the case of
phosphoacylglycerols.
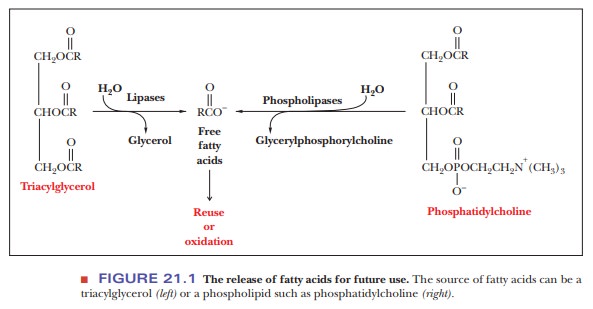
Several different phospholipases can be distinguished on the basis
of the site at which they hydrolyze phospholipids (Figure 21.2). Phospholipase
A2 is widely distributed in nature; it is also being actively studied
by biochemists interested in its structure and mode of action, which involves
hydrolysis of phospholipids at the surface of micelles. Phospholipase D occurs
in spider venom and is responsible for the tissue damage that accompanies
spider bites. Snake venoms also contain phospholipases; the concentration of
phospholipases is particularly high in venoms with comparatively low
concen-trations of the toxins (usually small peptides) that are characteristic
of some kinds of venom. The lipid products of hydrolysis lyse red blood cells,
prevent-ing clot formation. Snakebite victims bleed to death in this situation.
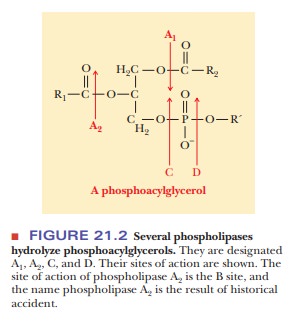
The release of fatty acids from triacylglycerols in adipocytes is controlled by hormones. In a scheme that will look familiar from our discussions of carbohy-drate metabolism, a hormone binds to a receptor on the plasma membrane of the adipocyte (Figure 21.3). This hormone binding activates adenylate cyclase, which leads to production of active protein kinase A (cAMP-dependent pro-tein kinase).

Protein kinase phosphorylates triacylglycerol
lipase, which cleaves the fatty acids from the glycerol backbone. The main
hormone that has this effect is epinephrine. Caffeine also mimics epinephrine
in this regard, which is one reason competitive runners often drink caffeine
the morning of a race. Distance runners want to burn fat more efficiently to
spare their carbohydrate stores for the later stages of the race.
How are fatty acids transported to the mitochondrion for oxidation?
Fatty-acid
oxidation begins with activation of
the molecule. In this reaction, a thioester bond is formed between the carboxyl
group of the fatty acid and the thiol group of coenzyme A (CoA-SH). The
activated form of the fatty acid is an acyl-CoA, the exact nature of which
depends on the nature of the fatty acid itself. Keep in mind throughout this
discussion that all acyl-CoA molecules are thioesters, since the fatty acid is
esterified to the thiol group of CoA.
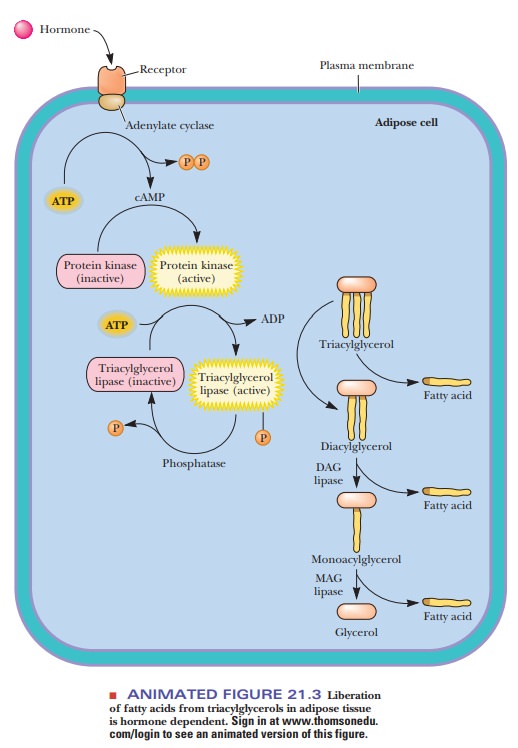
The
enzyme that catalyzes formation of the ester bond, an acyl-CoA synthetase, requires ATP for its action. In the course of
the reaction, an acyl adenylate intermediate is formed. The acyl group is then
transferred to CoA-SH. ATP is converted to AMP and PPi, rather than
to ADP and Pi. The PPi is hydrolyzed to two Pi;
the hydrolysis of two high-energy phosphate bonds provides energy for the
activation of the fatty acid and is equivalent to the use of two ATP. The
formation of the acyl-CoA is endergonic without the energy provided by the
hydrolysis of the two high-energy bonds. Note also that the hydrolysis of ATP
to AMP and two Pi represents an increase in entropy (Figure 21.4).
There are several enzymes of this type, some specific for longer-chain fatty
acids and some for shorter-chain fatty acids. Both saturated and unsaturated
fatty acids can serve as substrates for these enzymes. The esterification takes
place in the cytosol, but the rest of the reactions of fatty-acid oxidation
occur in the mito-chondrial matrix. The activated fatty acid must be
transported into the mito-chondrion so that the rest of the oxidation process
can proceed.
The acyl-CoA can cross the outer mitochondrial membrane but not the inner membrane (Figure 21.5). In the intermembrane space, the acyl group is transferred to carnitine by transesterification; this reaction is catalyzed by the enzyme carnitine acyltransferase, which is located in the inner membrane. Acyl-carnitine, a compound that can cross the inner mitochondrial membrane, is formed.
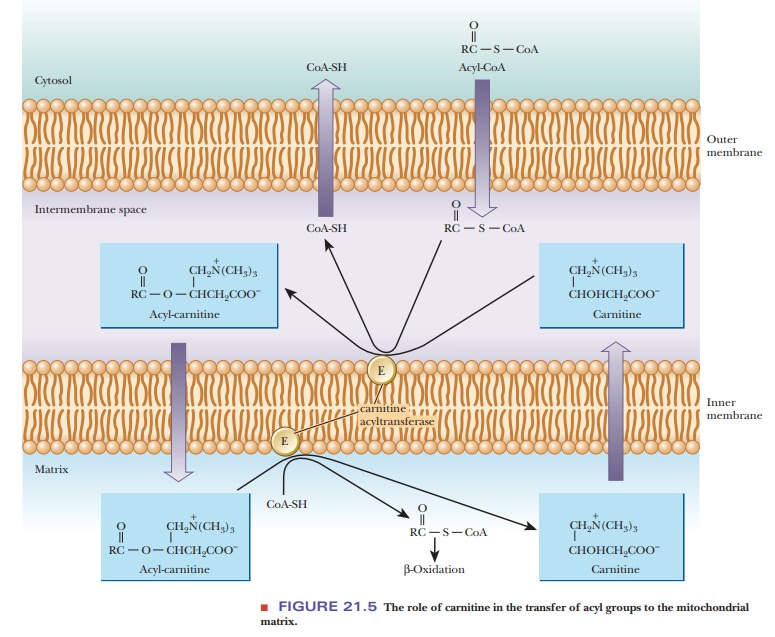
This enzyme has a specificity for acyl groups between 14 and 18 carbons
long and is often called carnitine
palmitoyltransferase (CPT-I) for this reason. The acyl-carnitine passes
through the inner membrane via a specific carnitine/acyl-carnitine transporter
called carnitine translocase. Once
in the matrix, the acyl group is transferred from carnitine to mitochondrial
CoA-SH by another transesterification reaction, involving a second carnitine
palmitoyl-transferase (CPT-II)
located on the inner face of the membrane.
How does oxidation of fatty acids take place?
In the matrix, a repeated sequence of reactions successively cleaves two-carbon units from the fatty acid, starting from the carboxyl end. This process is called β-oxidation,since the oxidative cleavage takes place at the β-carbon of the acylgroup esterified to CoA. The β-carbon of the original fatty acid becomes the carboxyl carbon in the next stage of degradation. The whole cycle requires four reactions (Figure 21.6).
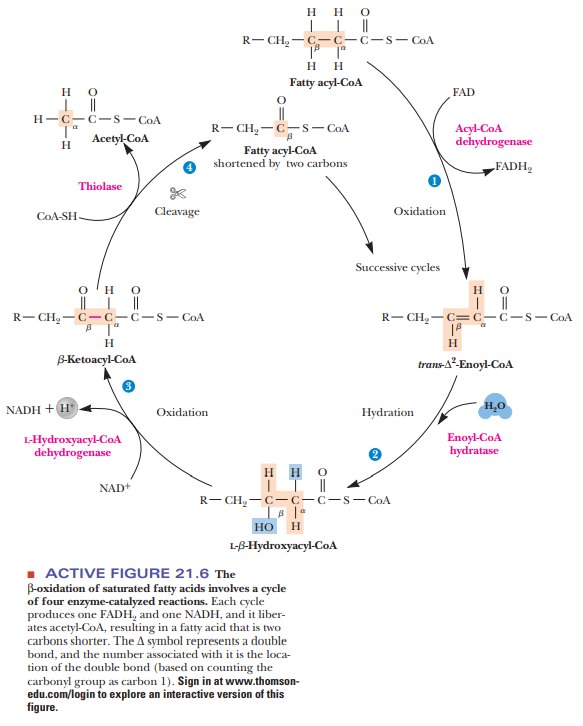
·
The acyl-CoA is oxidized to an α, β unsaturated acyl-CoA (also called a β-enoyl-CoA). The product has
the trans arrangement at the double
bond. This reaction is catalyzed by an FAD-dependent acyl-CoA dehydrogenase.
·
The unsaturated acyl-CoA is hydrated to produce a β-hydroxyacyl-CoA. This
reaction is catalyzed by the enzyme enoyl-CoA hydratase.
·
A second oxidation
reaction is catalyzed by β-hydroxyacyl-CoA dehydroge-nase, an NAD+-dependent
enzyme. The product is a β-ketoacyl-CoA.
·
The enzyme thiolase catalyzes the cleavage of the β-ketoacyl-CoA; a molecule of CoA is required
for the reaction. The products are acetyl-CoA and an acyl-CoA that is two
carbons shorter than the original molecule that entered the β-oxidation cycle. The CoA is
needed in this reaction to form the new thioester bond in the smaller acyl-CoA
molecule. This smaller molecule then undergoes another round of the β-oxidation cycle.
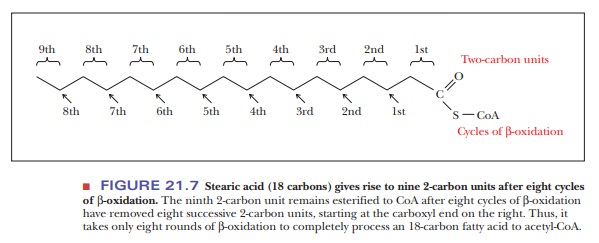
When a fatty acid with an even number of carbon atoms undergoes suc-cessive rounds of the β-oxidation cycle, the product is acetyl-CoA. (Fatty acids with even numbers of carbon atoms are the ones normally found in nature, so acetyl-CoA is the usual product of fatty-acid catabolism.) The number of mol-ecules of acetyl-CoA produced is equal to half the number of carbon atoms in the original fatty acid. For example, stearic acid contains 18 carbon atoms and gives rise to 9 molecules of acetyl-CoA. Note that the conversion of one 18-carbon stearic acid molecule to nine 2-carbon acetyl units requires eight, not nine, cycles of β-oxidation (Figure 21.7). The acetyl-CoA enters the citric acid cycle, with the rest of the oxidation of fatty acids to carbon dioxide and water taking place through the citric acid cycle and electron transport.
Recall
that most of the enzymes of the citric acid cycle are located in the
mitochondrial matrix, and we have just seen that the β-oxidation cycle takes place in the matrix as
well. In addi-tion to mitochondria, other sites of β-oxidation are known. Peroxisomes and
gly-oxysomes, organelles that carry out oxidation reactions, are also sites of β-oxidation, albeit to a far
lesser extent than the mitochondria. Certain drugs, called hypolipidemic drugs,
are used in an attempt to control obesity. Some of these work by stimulating β-oxidation in peroxisomes.
Summary
Fatty acids are activated and transported to the mitochondrial
matrix for further catabolism.
The breakdown of fatty acids takes place in the mitochondrial matrix and proceeds by successive removal of two-carbon units as acetyl-CoA. Each cleavage of a two-carbon moiety requires a four-step reaction sequence called β-oxidation.
Related Topics