Chapter: Biochemistry: Lipid Metabolism
Ketone Bodies
Ketone Bodies
Substances related to acetone (“ketone
bodies”) are produced when an excess of acetyl-CoA arises from β-oxidation. This condition
occurs when not enough oxaloacetate is available to react with the large
amounts of acetyl-CoA that could enter the citric acid cycle. Oxaloacetate in
turn arises from glycolysis because it is formed from pyruvate in a reaction catalyzed
by pyruvate carboxylase.
A situation like this can come about when an organism has a high
intake of lipids and a low intake of carbohydrates, but there are also other
possible causes, such as starvation and diabetes. Starvation conditions cause
an organ-ism to break down fats for energy, leading to the production of large
amounts of acetyl-CoA by β-oxidation. The amount of acetyl-CoA is
excessive by com-parison with the amount of oxaloacetate available to react
with it. In the case of people with diabetes, the cause of the imbalance is not
inadequate intake of carbohydrates but rather the inability to metabolize them.
Do acetone and acetyl-CoA have a connection in lipid metabolism?
The reactions that result in ketone bodies start with the condensation of two molecules of acetyl-CoA to produce acetoacetyl-CoA. Acetoacetate is produced from acetoacetyl-CoA through condensation with another acetyl-CoA to form β-hydroxy-β-methylglutaryl-CoA (HMG-CoA), a compound we will see againwhen we look at cholesterol synthesis (Figure 21.11). HMG-CoA lyase then releases acetyl-CoA to give acetoacetate.
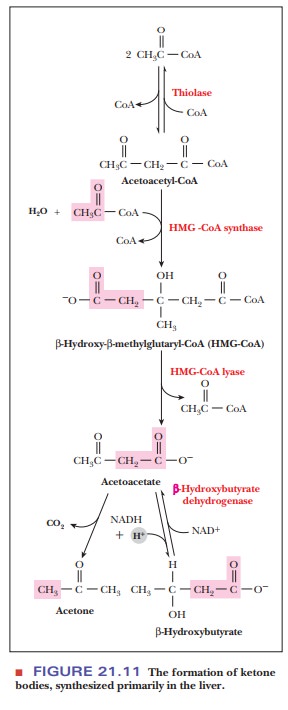
Acetoacetate can then have two fates. A
reduction reaction can produce β-hydroxybutyrate
from acetoacetate. The other possible reaction is the spontaneous
decarboxylation of acetoacetate to give acetone.
The odor of acetone can frequently be detected on the breath of people with
diabetes whose disease is not controlled by suitable treatment. The excess of
acetoacetate, and consequently of acetone, is a pathological condition known as
ketosis. Because acetoacetate and β-hydroxybutyrate are acidic, their presence at high concentration
overwhelms the buffering capacity of the blood. The body deals with the consequent
lowering of blood pH (ketoacidosis) by excreting H+ into the urine,
accompanied by excretion of Na+, K+, and water. Severe
dehydration can result (excessive thirst is a classic symptom of diabetes);
diabetic coma is another possible danger.
The principal
site of synthesis of ketone bodies is liver mitochondria, but they are not used
there because the liver lacks the enzymes necessary to recover acetyl-CoA from
ketone bodies. It is easy to transport ketone bodies in the bloodstream
because, unlike fatty acids, they are water-soluble and do not need to be bound
to proteins, such as serum albumin. Organs other than the liver can use ketone
bodies, particularly acetoacetate. Even though glucose is the usual fuel in
most tissues and organs, acetoacetate can be used as a fuel. In heart muscle
and the renal cortex, acetoacetate is the preferred source of energy.
Even in
organs such as the brain, in which glucose is the preferred fuel, starvation
conditions can lead to the use of acetoacetate for energy. In this situation,
acetoacetate is converted to two molecules of acetyl-CoA, which can then enter
the citric acid cycle. The key point here is that starvation gives rise to
long-term, rather than short-term, regulation over a period of hours to days
rather than minutes. The decreased level of glucose in the blood over a period
of days changes the hormone balance in the body, particularly involving
insu-lin and glucagon. (Short-term regulation, such as allosteric interactions
or covalent modification, can occur in a matter of minutes.) The rates of
protein synthesis and breakdown are subject to change under these conditions.
The specific enzymes involved are those involved in fatty-acid oxida-tion
(increase in levels) and those for lipid biosynthesis (decrease in levels).
Summary
If an organism has an excess of acetyl-CoA, it produces substances
related to acetone; thus the name “ketone bodies.”
This situation can arise from an excessive intake of fats compared
to car-bohydrates, or from diabetes.
Related Topics