Chapter: Biochemistry: Lipid Metabolism
Fatty-Acid Biosynthesis
Fatty-Acid Biosynthesis
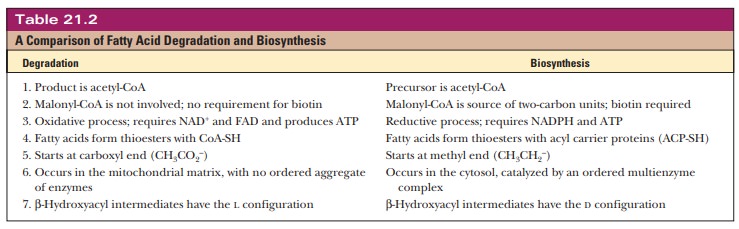
The anabolism of fatty acids is not simply a reversal of the
reactions of β-oxidation.
Anabolism and catabolism are not, in general, the exact reverse of each other;
for instance, gluconeogenesis is not
simply a reversal of the reactions of glycolysis. A first example of the
differences between the degradation and the biosynthesis of fatty acids is that
the anabolic reactions take place in the cytosol. We have just seen that the
degradative reactions of β-oxidation take place in the mitochondrial
matrix. The first step in fatty-acidbiosynthesis is transport of acetyl-CoA to
the cytosol.
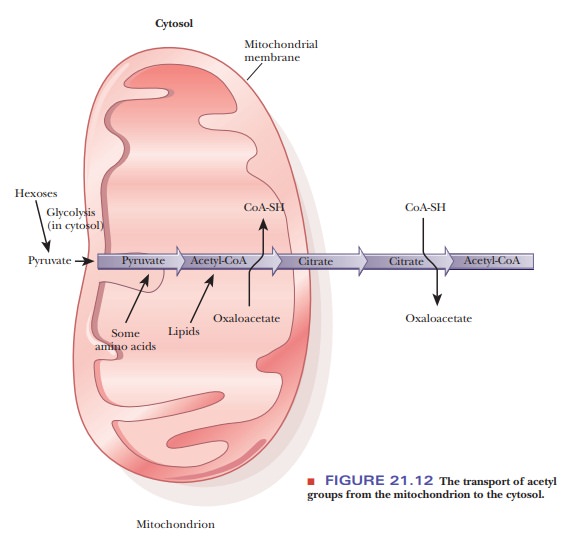
Acetyl-CoA can be formed either by β-oxidation of fatty acids or by decarbox-ylation of pyruvate. Most of these reactions take place in the mitochondria, requiring a transport mechanism to export acetyl-CoA to the cytosol for fatty- acid biosynthesis.
The transport mechanism is based on the fact
that citrate can cross the mitochondrial membrane. Acetyl-CoA condenses with
oxaloacetate, which cannot cross the mitochondrial membrane, to form citrate
(recall that this is the first reaction of the citric acid cycle).
How do the first steps of fatty-acid synthesis take place?
The
citrate that is exported to the cytosol can undergo the reverse reaction,
producing oxaloacetate and acetyl-CoA (Figure 21.12). Acetyl-CoA enters the
pathway for fatty-acid biosynthesis, while oxaloacetate undergoes a series of
reactions in which NADPH is substituted for NADH. This substitution controls
the pathway because NADPH is required for fatty-acid anabolism.
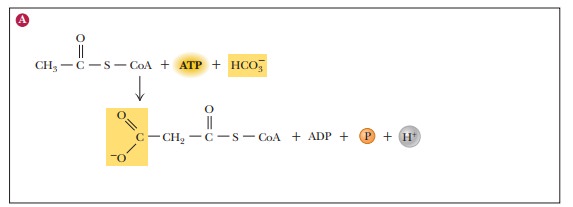
In the cytosol, acetyl-CoA is carboxylated, producing malonyl-CoA, a key intermediate in fatty-acid biosynthesis (Figure 21.13). This reaction is cata-lyzed by the acetyl-CoA carboxylase complex, which consists of three enzymes and requires Mn2+, biotin, and ATP for activity. We have already seen that enzymes catalyzing reactions that take place in several steps frequently consist of several separate protein molecules, and this enzyme follows that pattern. In this case, acetyl-CoA carboxylase consists of the three proteins biotin carboxylase, the biotincarrier protein, and carboxyl transferase. Biotin carboxylase catalyzes the transfer of the carboxyl group to biotin.
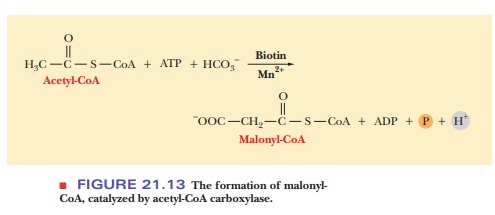
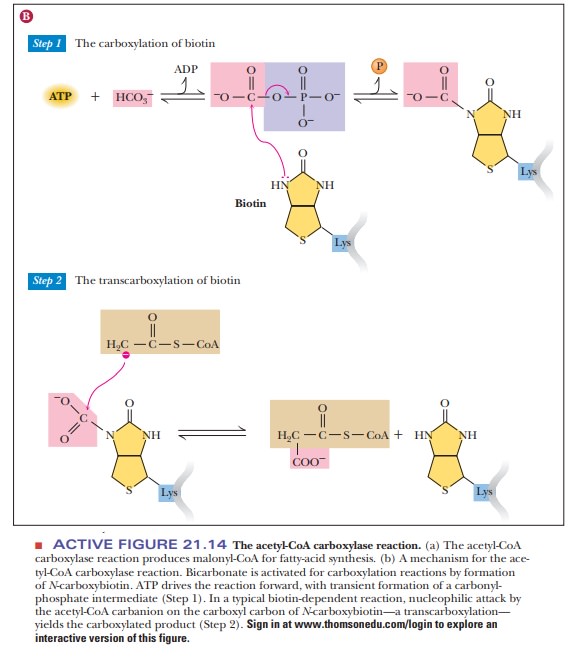
The “activated CO2” (the carboxyl group
derived from the bicarbonate ion HCO3–) is covalently
bound to biotin. Biotin (whether carboxylated or not) is bound to the biotin
carrier protein by an amide linkage to the ε-amino group of a lysine side chain. The amide linkage to the side
chain that bonds biotin to the carrier protein is long enough and flexible
enough to move the carboxylated biotin into position to transfer the carboxyl
group to acetyl-CoA in the reaction catalyzed by carboxyl transferase,
producing malonyl-CoA (Figure 21.14). In addition to its role as a starting
point in fatty-acid synthesis, malonyl-CoA strongly inhibits the carnitine
acyl-transferase I on the outer face of the inner mitochondrial membrane. This avoids
a futile cycle in which fatty acids are β-oxidized in the mitochondria to make acetyl-CoA just so they can
be remade into fatty acids in the cytosol.
What is the mode of action of fatty-acid synthase?
The
biosynthesis of fatty acids involves the successive addition of two-carbon
units to the growing chain. Two of the three carbon atoms of the malonyl group
of malonyl-CoA are added to the growing fatty-acid chain with each cycle of the
biosynthetic reaction. This reaction, like the formation of the malonyl-CoA
itself, requires a multienzyme complex located in the cytosol and not attached
to any membrane. The complex, made up of the individual enzymes, is called fatty-acid synthase.
The
usual product of fatty-acid anabolism is palmitate,
the 16-carbon satu-rated fatty acid. All 16 carbons come from the acetyl group
of acetyl-CoA; we have already seen how malonyl-CoA, the immediate precursor,
arises from acetyl-CoA. But first there is a priming step in which one molecule
of acetyl-CoA is required for each molecule of palmitate produced. In this
priming step, the acetyl group from acetyl-CoA is transferred to an acyl carrier protein (ACP), which is considered
a part of the fatty-acid synthase complex (Figure 21.15). The acetyl group is
bound to the protein as a thioester. The group on the pro-tein to which the
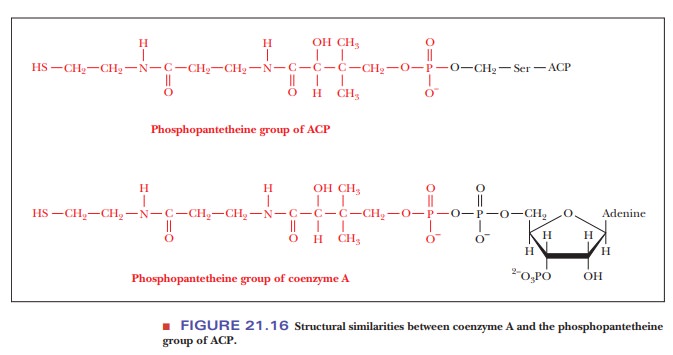
acetyl group is bonded is the 4-phosphopantetheine group, which in turn is
bonded to a serine side chain; note in Figure 21.16 that this group is
structurally similar to CoA-SH itself. The acetyl group is transferred from
CoA-SH, to which it is bound by a thioester linkage, to the ACP; the ace-tyl
group is bound to the ACP by a thioester linkage. Although the functional group
of ACP is similar to that of CoA-SH, it is noteworthy that fatty-acid
synthe-sis in the cytosol uses only ACP. In essence, the ACP is a label that
marks acetyl groups for fatty-acid synthesis.
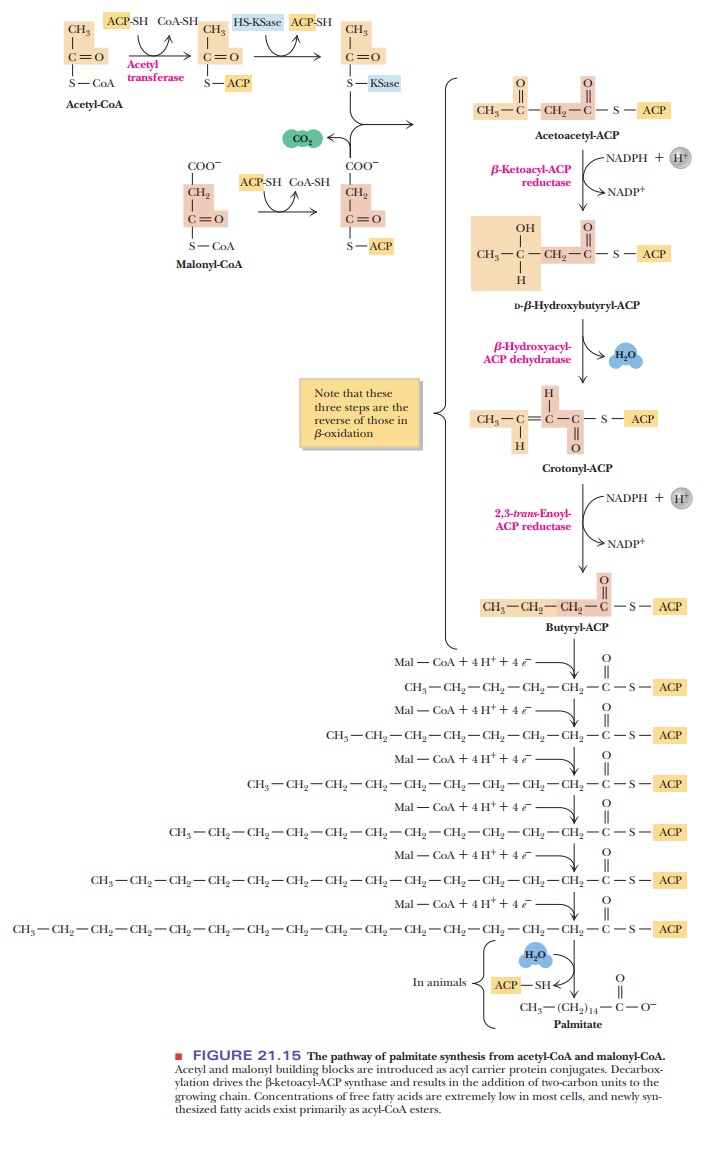
The
acetyl group is transferred in turn from the ACP to another protein, to which
it is bound by a thioester linkage to a cysteine-SH; the other protein is β-ketoacyl-S-ACP-synthase
(HS-KSase) (Figure 21.15). The first of the successiveadditions of two of the
three malonyl carbons to the fatty acid starts at this point. The malonyl group
itself is transferred from a thioester linkage with CoA-SH to another thioester
bond with ACP (Figure 21.15). The next step is a condensa-tion reaction that
produces acetoacetyl-ACP (Figure 21.15). In other words, the principal product
of this reaction is an acetoacetyl group bound to the ACP by a thioester
linkage. Two of the four carbons of acetoacetate come from the priming acetyl
group, and the other two come from the malonyl group. The carbon atoms that
arise from the malonyl group are the one directly bonded to the sulfur and the
one in the —CH2— group next to it. The CH3CO— group comes
from the priming acetyl group. The other carbon of the malonyl group is
released as CO2; the CO2 that is lost is the original CO2
that was used to carboxylate the acetyl-CoA to produce malonyl-CoA. The
synthase is no longer involved in a thioester linkage. This is an example of a
decarboxylation being used to drive an otherwise unfavorable condensation
reaction.
Acetoacetyl-ACP
is converted to butyryl-ACP by a series of reactions involving two reductions
and a dehydration (Figure 21.15). In the first reduction, the β-keto group is reduced to an alcohol, giving rise toD-β-hydroxybutyryl-ACP.In the process, NADPH is oxidized to NADP+;
the enzyme that catalyzes this reaction is β-ketoacyl-ACP reductase (Figure 21.15). The dehydration step,
catalyzed by β-hydroxyacyl-ACP dehydratase, produces
crotonyl-ACP (Figure 21.15). Note that the double bond is in the trans configuration. A second reduction
reaction, catalyzed by β-enoyl-ACP reductase, produces butyryl-ACP
(Figure 21.15). In this reaction, NADPH is the coenzyme, as it was in the first
reduction reaction in this series.
In the second round of fatty-acid biosynthesis, butyryl-ACP plays the same role as acetyl-ACP in the first round. The butyryl group is transferred to the synthase, and a malonyl group is transferred to the ACP. Once again there is a condensation reaction with malonyl-ACP (Figure 21.15). In this second round, the condensation produces a six-carbon β-ketoacyl-ACP. The two added carbon atoms come from the malonyl group, as they did in the first round. The reduc-tion and dehydration reactions take place as before, giving rise to hexanoyl-ACP. The same series of reactions is repeated until palmitoyl-ACP is produced. In mammalian systems, the process stops at C16 because the fatty-acid synthase does not produce longer chains. Mammals produce longer-chain fatty acids by modifying the fatty acids formed by the synthase reaction.
Fatty-acid
synthases from different types of organisms have markedly dif-ferent
characteristics. In Escherichia coli,
the multienzyme system consists of an aggregate of separate enzymes, including
a separate ACP. The ACP is of pri-mary importance to the complex and is
considered to occupy a central position in it. The phosphopantetheine group
plays the role of a “swinging arm,” much like that of biotin, which was
discussed earlier. This bacterial system has been extensively studied and has
been considered a typical example of a fatty-acid synthase. In eukaryotes,
however, fatty-acid synthesis occurs on a multienzyme complex. In yeast, this
complex consists of two different types of subunits, called α and β, arranged in anα6β6 complex. In mammals, the fatty-acid synthase
contains only one type of subunit, but the active enzyme is a dimer of this
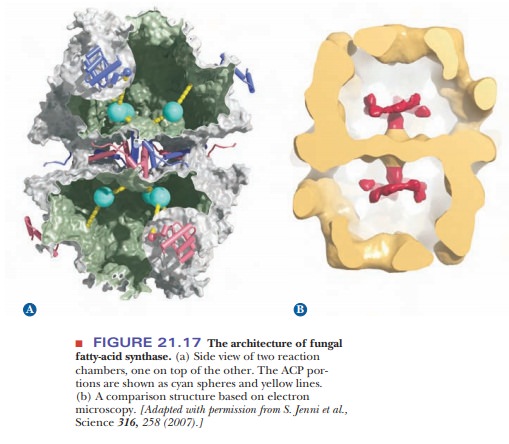
single subunit. It has been determined by X-ray crystallography that the
mammalian synthase contains two reaction chambers in which the vari-ous
components are held in proximity to each other as the reaction proceeds. Each
of the subunits is a multifunctional
enzyme that catalyzes reactions requiring several different proteins in the
E. coli system. The structure of
fungal fatty-acid synthase was recently elucidated in even greater detail by
X-ray crystallography. The results demonstrated that the multiple active sites
for the synthase reac-tions are arranged in the reaction chamber so that a
circular movement of the ACP-bound substrates can deliver the substrate to each
specific active site (Figure 21.17). The growing fatty-acid chain swings back
and forth between enzyme activities contained on different subunits, using ACP
as a “swinging arm.” Like the bacterial system, the eukaryotic system keeps all
the components of the reaction in proximity to one another, which shows us
another example of the advantages to multienzyme complexes. For more
information about the structure of eukaryotic synthases.
Several additional reactions are required for the elongation of fatty-acid chains and the introduction of double bonds. When mammals produce fatty acids with longer chains than that of palmitate, the reaction does not involve cytosolic fatty-acid synthase. There are two sites for the chain-lengthening reactions: the endoplasmic reticulum (ER) and the mitochondrion. In the chain-lengthening reactions in the mitochondrion, the intermediates are of the acyl-CoA type rather than the acyl-ACP type. In other words, the chain-lengthening reactions in the mitochondrion are the reverse of the catabolic reactions of fatty acids, with acetyl-CoA as the source of added carbon atoms; this is a difference between the main pathway of fatty-acid biosynthesis and these modification reactions. In the ER, the source of additional carbon atoms is malonyl-CoA. The modification reactions in the ER also differ from the bio-synthesis of palmitate in that, like the mitochondrial reaction, no intermediates are bound to ACP.
Reactions
in which a double bond is introduced in fatty acids mainly take place on the
ER. The insertion of the double bond is catalyzed by a mixed-function oxidase
that requires molecular oxygen (O2) and NAD(P)H. During the
reaction, both NAD(P)H and the fatty acid are oxidized, while oxygen is reduced
to water. Reactions linked to molecular oxygen are comparatively rare. Mammals
cannot introduce a double bond beyond carbon atom 9 (counting from the carboxyl
end) of the fatty-acid chain. As a result, linoleate [CH3—(CH2)4—CH=CH—CH2—CH=CH—(CH2)7—COO–],
with two double bonds, and linolenate [CH3—(CH2)4—CH=CH—CH2CH=CH—
CH2—CH=CH—(CH2)4COO–], with three
double bonds, must be included in the diets of mammals. They are essential fatty acids because they are
precursors of other lipids, including prostaglandins.

Even though both the anabolism and the catabolism of fatty acids require successive reactions of two-carbon units, the two pathways are not the exact reversal of each other. The differences between the two pathways are summa-rized in Table 21.2. The sites in the cell in which various anabolic and catabolic reactions take place are shown in Figure 21.18.
Summary
Acetyl-CoA is transported to the cytosol and converted to
malonyl-CoA. Chain lengthening takes place in the cytosol as well.
The biosynthesis of fatty acids proceeds by the addition of
two-carbon units to the hydrocarbon chain. The process is catalyzed in many
organ-isms by a large multienzyme complex called fatty-acid synthase.
Related Topics