Chapter: Biochemistry: Lipid Metabolism
Catabolism of Unsaturated Fatty Acids and Odd-Carbon Fatty Acids
Catabolism of Unsaturated Fatty
Acids and Odd-Carbon Fatty Acids
Fatty acids with odd numbers of carbon atoms are not as frequently
encountered in nature as are the ones with even numbers of carbon atoms.
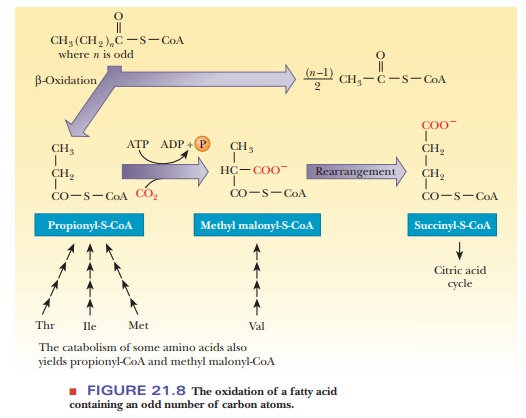
Odd-numbered fatty acids also undergo β-oxidation (Figure 21.8). The last cycle of β-oxidation produces one
molecule of propionyl-CoA. An enzymatic pathway exists to convert propionyl-CoA
to succinyl-CoA, which then enters the citric acid cycle. In this pathway,
propionyl-CoA is first carboxylated to methyl malonyl-CoA in a reaction
catalyzed by propionyl-CoA carboxylase, which then undergoes rearrangement to
form succinyl-CoA. Because propionyl-CoA is also a product of the catabolism of
several amino acids, the conversion of propionyl-CoA to succinyl-CoA also plays
a role in amino acid metabolism. The conversion of methyl malonyl-CoA to
succinyl-CoA requires vitamin B12 (cyanocobalamin), which has
a cobalt(III) ion in its active state.
How does the oxidation of unsaturated fatty acids differ from that of saturated fatty acids?
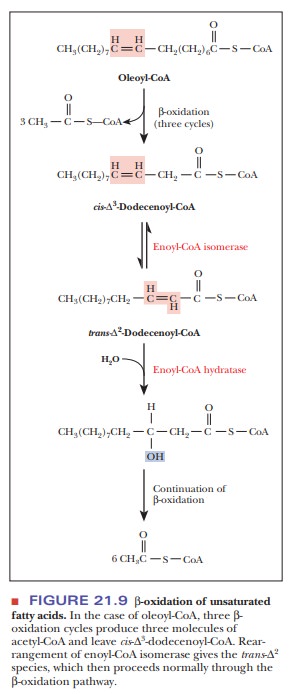
The conversion of a monounsaturated fatty acid to acetyl-CoA
requires a reaction that is not encountered in the oxidation of saturated
acids, a cis–trans isomerization
(Figure 21.9). Successive rounds of β-oxidation of oleic acid (18:1) provide an
example of these reactions. The process of β-oxidation gives rise to unsaturated fatty
acids in which the double bond is in the trans
arrangement,
whereas the double bonds in most naturally occurring fatty acids are in the cis arrangement. In the case of oleic
acid, there is a cis double bond
betweencarbons 9 and 10. Three rounds of β-oxidation produce a 12-carbon unsaturated fatty acid with a cis double bond between carbons 3 and 4.
The hydratase of the β-oxidation cycle requires atransdouble bond between carbon atoms 2 and3 as a substrate. A cis–transisomerase produces a trans
double bond between carbons 2 and 3 from the cis double bond between carbons 3 and 4. From this point forward,
the fatty acid is metabolized the same as for saturated fatty acids. When oleic
acid is β-oxidized, the first step (fatty acyl-CoA
dehydrogenase) is skipped, and the isomerase deals with the cis double bond, putting it into the
proper position and orientation to continue the pathway.
When polyunsaturated fatty acids are β-oxidized, another enzyme is needed to handle the second double bond. Let’s consider how linoleic acid (18:2) would be metabolized (Figure 21.10). This fatty acid has cis double bonds at positions 9 and 12 as shown in Figure 21.10, which are indicated as cis- 9 and cis-12. Three normal cycles ofβ-oxidation occur, as in our example with oleicacid, before the isomerase must switch the position and orientation of the double bond.
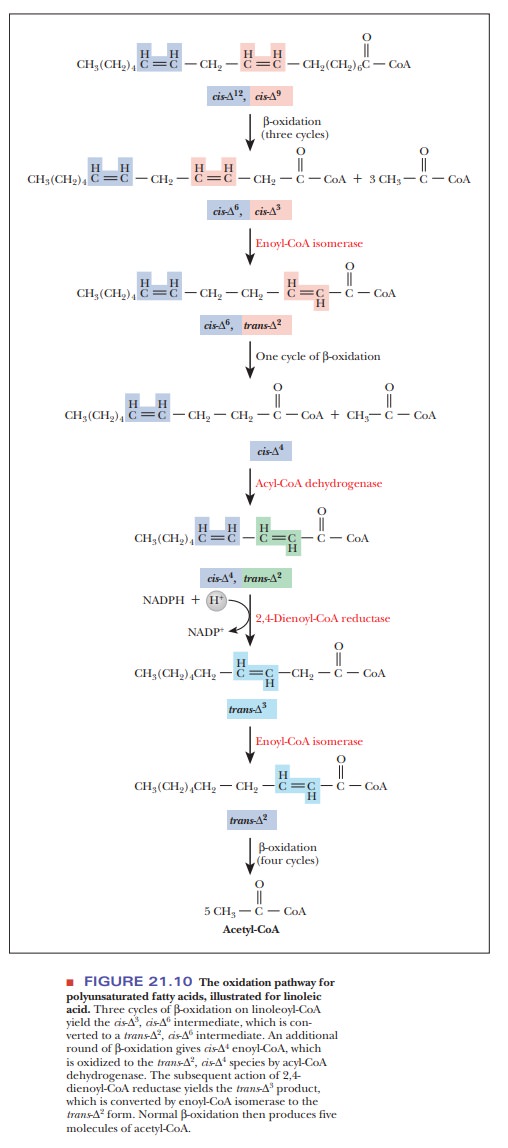
The cycle of β-oxidation continues until a 10-carbon fatty
acyl-CoA is attained that has one cis
double bond on its carbon 4 (cis- 4).
Then the first step of β-oxidation occurs, putting in a trans double bond between carbons 2 and
3 (α and β). Normal β-oxidation cannot continue at this point because the fatty acid
with the two double bonds so close together is a poor substrate for the
hydratase. Therefore, a second new enzyme, 2,4-dienoyl-CoA
reductase, uses NADPH to reduce this intermediate. The result is a fatty
acyl-CoA with a trans double bond
between carbons 3 and 4. The isomerase then switches the trans double from carbon 3 to carbon 2, and β-oxidation continues.
A
molecule with three double bonds, such as linolenic acid (18:3), would use the
same two enzymes to handle the double bonds. The first double bond requires the
isomerase. The second one requires the reductase and the isom-erase, and the
third requires the isomerase. For practice, you can diagram the β-oxidation of an 18-carbon molecule with cis double bonds at positions 9, 12, and 15 to see that this is
true. Unsaturated fatty acids make up a large enough portion of the fatty acids
in storage fat (40% for oleic acid alone) to make the reactions of the cis–trans isomerase and the epimerase of
particular importance.
The
oxidation of unsaturated fatty acids does not generate as many ATPs as it would
for a saturated fatty acid with the same number of carbons. This is because the
presence of a double bond means that the acyl-CoA dehydroge-nase step will be
skipped. Thus, fewer FADH2 will be produced.
Summary
Fatty acids with uneven numbers of carbon atoms produce
propionyl-CoA in the last round of β-oxidation. Propionyl-CoA can be converted to
succinyl-CoA, which plays a role in the citric acid cycle.
The oxidation of unsaturated fatty acids requires enzymes that
catalyze isomerization around the double bonds so that oxidation can proceed.
Related Topics