Chapter: Introduction to Human Nutrition: Minerals and Trace Elements
Zinc: Toxicity, Genetic diseases, Requirements, dietary sources, Micronutrient interactions
Zinc
The natural abundance of zinc in the Earth’s crust is 0.02%. The principal ores of zinc are sphalerite or blende (sulfide), smithsonite (carbonate), calamine (silicate), and franklinite (zinc iron oxide). Zinc is used to form numerous alloys with other metals.
Brass, nickel, silver, typewriter metal, commercial bronze, spring brass, German silver, soft solder, and aluminum solder are some of the more important alloys. Large quantities of zinc are used to produce die castings, used extensively by the automotive, electri-cal, and hardware industries. Zinc is also extensively used to galvanize other metals, such as iron to prevent corrosion. Zinc oxide is widely used in the manufac-ture of paints, rubber products, cosmetics, pharma-ceuticals, floor coverings, plastics, printing inks, soap, storage batteries, textiles, electrical equipment, and other products. Zinc sulfide is used in making lumi-nous dials, X-ray and television screens, and fluores-cent lights. The chloride and chromate are also important compounds. In biological systems zinc is virtually always in the divalent (+2) state. Unlike iron, zinc does not exhibit any direct redox chemistry.
Toxicity
Gross acute zinc toxicity has been described following the drinking of water that has been stored in galva-nized containers or the use of such water for renal dialysis. Symptoms include nausea, vomiting, and fever, and are apparent after acute ingestion of 2 g or more. The more subtle effects of moderately elevated intakes, not uncommon in some populations, are of greater concern, because they are not easily detected. Prolonged intakes of supraphysiological intakes of zinc (75–300 mg/day) have been associated with impaired copper utilization (producing features such as microcytic anemia and neutropenia), impaired immune responses and a decline of high-density lipo-proteins, but some have argued that even short-term intakes of about 25–50 mg zinc/day may interfere with the metabolism of both iron and copper. The US Food and Nutrition Board reported that there was no evidence of adverse effects from intake of naturally occurring zinc in food; however, they derived a toler-able UL of 40 mg/day for adults older than 19 years, which applies to total zinc intake from food, water, and supplements (including fortified foods). Data on reduced copper status in humans were used to derive this UL for zinc. Using similar data but different uncertainty factors, the UL for total zinc intake was set at 25 mg/day in the EU.
Genetic diseases
Acrodermatitis enteropathica, a rare, inborn, auto-somal recessive disease, is a disorder of primary zinc malabsorption. It is characterized by alopecia; vesicu-lar, pustular and/or eczematoid skin lesions, specifi-cally of the mouth, face, hands, feet and groin; growth retardation; mental apathy; diarrhea and secondary malabsorption, defects in cellular and phagocytic immune function; and intercurrent infections. The disorder responds very well to zinc therapy.
Assessing status
Measurement of zinc in plasma or activities of zinc metalloenzymes or peptides in blood are frequently used to measure zinc status. They are not ideal indices, however, as they are relatively resistant to changes in dietary zinc and, moreover, metabolic conditions unrelated to zinc status cause them to decline. The development of zinc deficiency is different from that of many other nutrients because a functional reserve or store of zinc does not seem to be available when zinc intake is inadequate. Tissue zinc is conserved by reduction or cessation of growth in growing organ-isms or by decreased excretion in nongrowing organ-isms. Depending on the degree of deficiency, zinc homeostasis can be re-established by adjusting growth and excretion or, with a more severe deficiency, further metabolic changes occur, resulting in a negative zinc balance and loss of tissue zinc.
Requirements and dietary sources
The US RDA for zinc was based primarily on data derived from metabolic balance studies. Such studies are technically difficult to perform and it is uncertain whether information from these studies reflects true requirements. A different approach, using the factorial method, was proposed for estimates of zinc requirements and future RDAs. Factorial calculations to estimate zinc requirements require knowledge of obligatory losses, tissue composition, and needs for growth and tissue repair. Current RDAs for zinc (rec-ommended by the US Food and Nutrition Board in 2001) are infants 2 mg [first 6 months; this is an ade-quate intake (AI) value], 3 mg (7–12 months), chil-dren 3 and 5 mg (1–3 and 4–8 years, respectively), teenage boys 8 and 11 mg (9–13 and 14–18 years, respectively), adult men 11 mg (19 years and more), teenage girls 8 and 9 mg (9–13 and 14–18 years, respectively), adult women 8 mg (19 years and older), pregnant women 13 and 11 mg (younger than 18 years and 19–50 years, respectively) and lactating women 14 and 12 mg (younger than 18 years and 19–50 years, respectively).
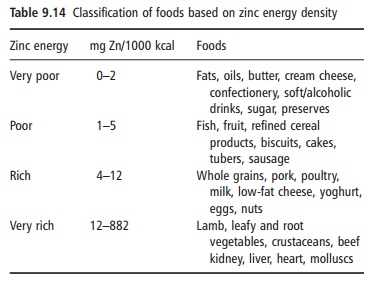
The zinc content of some common foods is given in Table 9.13, whereas Table 9.14 classifies foods based on zinc energy density. The bioavailability of zinc in different foods varies widely, from 5% to 50%. Meat, seafood (in particular oysters) and liver are good sources of bioavailable zinc. It has been estimated that approximately 70% of dietary zinc in the US diet is provided by animal products. In meat products, the zinc content to some extent follows the color of the meat, so that the highest content, approximately 50 mg/kg, is found in lean red meat, at least twice that
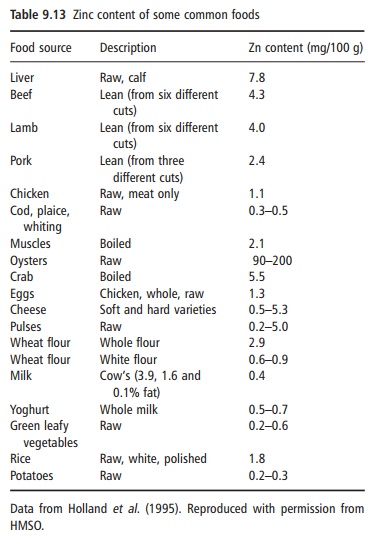
in chicken. However, in many parts of the world, most zinc is provided by cereals. In cereals, most of the zinc is found in the outer fiber-rich part of the kernel. The degree of refinement, therefore, determines the total zinc content. Wholegrain products provide 30–50 mg/ kg, but a low extraction rate wheat flour contains 8–10 mg/kg. The bioavailability of zinc can be low from plant-based diets, in particular from wholegrain cereals and legumes, owing to the high content of phytic acid, a potent inhibitor of zinc absorption.
Micronutrient interactions
A decrease in copper absorption has been reported in the presence of excessive zinc. Data indicate that the level necessary to impair bioavailability is >40–50 mg/ day; therapeutic levels (150 mg/day) over extended periods produce symptoms of copper deficiency. As mentioned above, iron under certain circumstances impairs zinc absorption. Animal studies have sug-gested an interaction between calcium and zinc in
Related Topics