Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
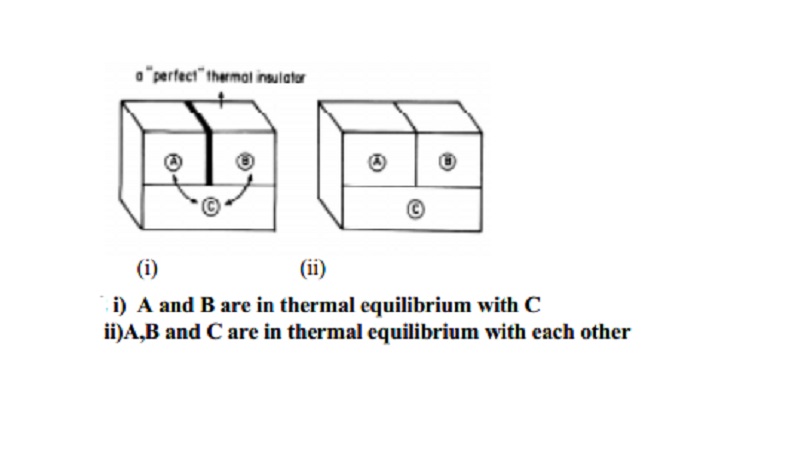
Zeroth law of thermodynamics or Thermal equilibrium
Zeroth law of thermodynamics
Consider
any two objects each maintained at different temperature, when brought in
thermal contact with each other such that heat is exchanged until a thermal
equilibrium is reached, then the two objects are considered to have equal
temperatures. For example, if a beaker containing water and a thermometer are
the two objects, while reading the temperature of the water in the beaker using
the thermometer, a thermal equilibrium is reached between the two objects
having a contact with each other. Also, when the temperatures of the
thermometer bulb and that of water in the beaker are same, thermal equilibrium
has said to be occurred.
Zeroth law of thermodynamics is also known as the law of thermal equilibrium. It provides a logical
basis for the concept of temperature of a system. It can be stated as follows.
`If two systems at different temperatures are separately in thermal
equilibrium with a third one, then they tend to be in thermal equilibrium with
themselves'.
Conversely, the Zeroth law can be stated in another manner as,
Related Topics