Surface Chemistry - Zeolite Catalysis | 12th Chemistry : UNIT 10 : Surface Chemistry
Chapter: 12th Chemistry : UNIT 10 : Surface Chemistry
Zeolite Catalysis
Zeolite Catalysis:
The details of heterogeneous catalysis will be incomplete, if zeolites
are not discussed. Zeolites are microporous, crystalline, hydrated, alumino
silicates, made of silicon and aluminium tetrahedron. There are about 50
natural zeolites and 150 synthetic zeolites. As silicon is tetravalent and
aluminium is trivalent, the zeolite matrix carries extra negative charge. To
balance the negative charge, there are extra framework cations for example, H+
or Na+ ions. Zeolites carrying protons are used as solid acid
catalysts and they are extensively used in the petrochemical industry for
cracking heavy hydrocarbon fractions into gasoline, diesel,etc., Zeolites
carrying Na+ ions are used as basic catalysts.
One of the most important applications of zeolites is their shape
selectivity. In zeolites, the active sites namely protons are lying inside
their pores. So, reactions occur only inside the pores of zeolites.
Reactant selectivity:
When bulkier molecules in a reactant mixture are prevented from reaching
the active sites within the zeolite crystal, this selectivity is called
reactant shape selectivity.
Transition state selectivity:
If the transition state of a reaction is large compared to the pore size
of the zeolite, then no product will be formed.
Product selectivity:
It is encountered when certain product molecules are too big to diffuse
out of the zeolite pores.
Phase Transfer catalysis:
Suppose the reactant of a reaction is present in one solvent and the
other reactant is present in an another solvent. The reaction between them is
very slow, if the solvents are immiscible. As the solvents form separate
phases, the reactants have to migrate across the boundary to react. But
migration of reactants across the boundary is not easy. For such situations a
third solvent is added which is miscible with both. So, the phase boundary is
eliminated, reactants freely mix and react fast. But for large scale production
of any product, use of a third solvent is not convenient as it may be
expensive. For such problems phase transfer catalysis provides a simple
solution, which avoids the use of solvents. It directs the use a phase transfer
catalyst (a phase transfer reagent) to facilitate transport of a reactant in
one solvent to the other solvent where the second reactant is present. As the
reactants are now brought together, they rapidly react and form the product.
Example:
Substitution of Cl- and CN– in the following reaction.
R-Cl + NaCN → R-CN + NaCl
organic phase + aqueous phase → organic phase + aquueous phase
R-Cl=1-chlorooctane
R-CN=1-cyanooctane
By direct heating of two phase mixture of organic 1-chlorooctane with
aqueous sodium cyanide for several days, 1-cyanooctane is not obtained.
However, if a small amount of quaternary ammonium salt like
tetraalkylammoniumchloride is added, a rapid transition of 1-cyanooctane occurs
in about 100% yield after 1 or 2 hours. In this reaction, the
tetraalkylammonium cation, which has hydrophobic and hydrophilic ends,
transports CN-from the aqueous phase to the organic phase using its
hydrophilic end and facilitates the reaction with 1-chloroocatne as shown
below:
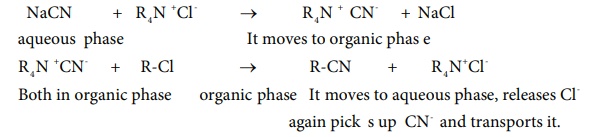
NaCN + R4 N + Cl- → R4 N + CN- + NaCl
aqueous phase → It moves
to organic phas e
R4 N + CN- + R-Cl → R-CN + R4 N+
Cl-
Both in organic phase ; organic phase ; It moves to aqueous phase,
releases Cl- again pick s up CN- and transports it.
So phase transfer catalyst, speeds up the reaction by transporting one
reactant from one phase to another.
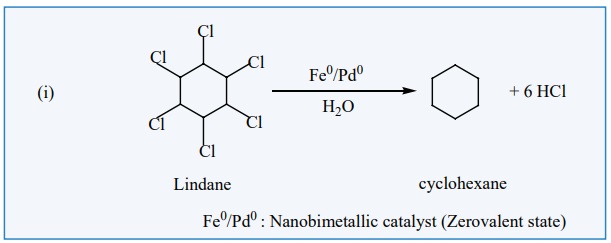
Nano Catalysis:
Nano materials such a metallic nano particles, metal oxides, etc., are
used as catalyst in many chemical transformation, Nanocatalysts carry the
advantages of both homogeneous and heterogeneous catalyses. Like homogeneous
catalysts, the nanocatalysts give 100% selective transformations and excellent
yield and show extremely high activity. Like the heterogeneous catalysts,
nanocatalysts can be recovered and recycled. Nanocatalysts are actually soluble
heterogeneous catalysts. An example for nanoparticles catalysed reaction is
given below
Related Topics