Surface Chemistry - Definition and types of Catalysis | 12th Chemistry : UNIT 10 : Surface Chemistry
Chapter: 12th Chemistry : UNIT 10 : Surface Chemistry
Definition and types of Catalysis
Catalysis
In 1836 Berzelius identified certain substances loosen the bond in the reacting molecules and increased the rate of the reaction. But he also found these substances didn’t undergo any change chemically. In order to indicate the property, he gave them the name catalyst. (In greek, kata-wholly, lein-to loosen).
Later it was identified that there were many substances which retarded the speed of a reaction.
Hence a catalyst is defined as a substance which alters the rate of chemical reaction without itself undergoing chemical change. The phenomenon which involves the action of a catalyst is called catalysis.
Positive and negative catalysis:
In positive catalysis, the rate of a reaction is increased by the presence of catalyst but in negative catalysis, the rate of reaction is decreased by the presence of a catalyst.
The two main types of catalysis (i) Homogeneous catalysis and (ii) Heterogeneous catalysis
Homogeneous catalysis
In a homogeneous catalysed reaction, the reactants, products and catalyst are present in the same phase.
Illustration (1):
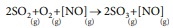
In this reaction the catalyst NO, reactants, SO2 and O2 , and product, SO3 are present in the gaseous form.
Illustration (2):
In the decomposition of acetaldehyde by I2 catalyst, the reactants and products are all present in the vapour phase.
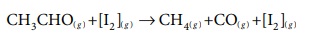
Let us consider some examples in which the reactants, products and catalyst are present in aqueous solution.
(1) Hydrolysis of cane sugar with a mineral acid as catalyst
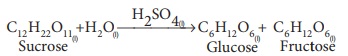
(2) Ester hydrolysis with acid or alkali as catalyst
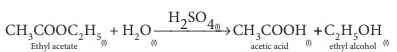
Heterogeneous catalysis
In a reaction, the catalyst is present in a different phase i.e. it is not present in the same phase as that of reactants or products. This is generally referred as contact catalysis and the catalyst present is in the form of finely divided metal or as gauze
Illustration
i) In the manufacture of sulphuric acid by contact process SO3 is prepared by the action of SO2 and O2 in the presence of Pt or V2O5 as a catalyst.
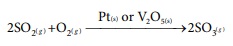
ii) In the Haber’s process for the manufacture of ammonia, iron is used as a catalyst for the reaction between Hydrogen and Nitrogen.
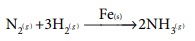
iii) Oxidation of ammonia is carried out in presence of platinum gauze
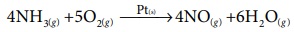
iv) The hydrogenation of unsaturated organic compounds is carried out using finely divided nickel as a catalyst.
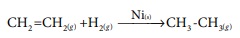
v) Decomposition of H2O2 occurs in the presence of the Pt catalyst
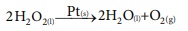
vi) In the presence of anhydrous AlCl3, benzene reacts with ethanoyl chloride to produce acetophenone

Related Topics